Advertisements
Jacquard Procion MX Dye

This book explains the use of Procion MX dyes.
Fabric Painting and Dyeing

Dyes and Paints


Advertisements
Jacquard Procion MX Dye

Fabric Painting and Dyeing

Dyes and Paints


One of the most beloved of all dyes anywhere that are used for hand dyeing is the beautiful turquoise found in the dichlorotriazine line of dyes, which are popularly known as Procion MX dyes, or simply MX dyes. It is so close to a true cyan, as used in the printing scheme of CYMK, that it is nearly ideal for color mixing, far superior to any redder shade of blue. When you select your mixing primaries, using a pure cyan, instead of royal blue, allows you to mix brighter, purer colors, though you can aways also add a bit of red for results identical to starting with a royal blue.
It's also one of the most finicky of dyes in that dye class. Whenever someone comes to me with a problem of low reaction temperatures, the main symptom is always that their turquoises are pale. A studio temperature that cerulean or fuchsia or golden yellow will take in stride makes turquoise too weak to work. It must have a reaction temperature of at least 70°F (21°C) to dye reliably, and warmer than that is better. The answer to the problem is easy: find a warm place to batch-cure your dyeing.
The greater sensitivity to a cold studio reflects turquoise's lower reactivity. This also causes it to react with fiber more slowly. In tie-dyeing soda-soaked garments, this means that you often see blue halos around other colors, as the result of separation of mixed colors on the fabric.
For scientific use, turquoise MX-G turns out to be a problem. Unlike the other popular dichlorotriazine dyes, it does not stay the same color! When you first mix some fresh dye powder into water, it's close to a pure turquoise in color, but if you let it sit around a while, it actually changes in color to become more blue!
If you check the absorption spectrum for Turquoise MX-G, which is a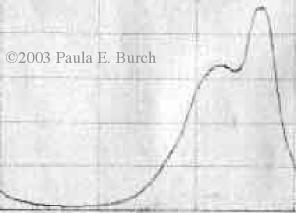 graph showing how much of the light is absorbed for each color wavelength in the rainbow, you will see that there is not just a single peak, as you'd expect for such a bright, clear dye color, but instead there are two - see the picture at the right. (The graph shows the spectrum of light absorption from violet at the left, through blue, then green, then yellow, orange, and finally red to the right; the color you see is the opposite of the color that is absorbed.) The rightmost peak is the one that creates a cyan color; it absorbs reddish-orange light. The peak to its left creates a bluer color; it absorbs more orange and yellow wavelengths. There is some overlap between the two peaks.
graph showing how much of the light is absorbed for each color wavelength in the rainbow, you will see that there is not just a single peak, as you'd expect for such a bright, clear dye color, but instead there are two - see the picture at the right. (The graph shows the spectrum of light absorption from violet at the left, through blue, then green, then yellow, orange, and finally red to the right; the color you see is the opposite of the color that is absorbed.) The rightmost peak is the one that creates a cyan color; it absorbs reddish-orange light. The peak to its left creates a bluer color; it absorbs more orange and yellow wavelengths. There is some overlap between the two peaks.
When looking for a good cyan dye to use for his research, Dr. François Denis found that the relative amount of the cyan and blue peaks changed within days of preparing the dye solution. The color shifted noticeably to become bluer, less cyan. (See his post in the Dye Forum for July 27, 2006.) We suspect the same may occur more slowly even in undissolved dye, as the dye powder ages. When he filtered the dye molecules by size, he found that the blue peak was almost entirely elimated at a molecular size below 1000 Daltons. The blue peak is apparently composed of aggregates of two or more molecules of the turquoise dye, perhaps five or ten particles. Click on the image below to see the full-sized graph....
Dharma Trading Company says that more intense results can be obtained in vat-dyeing with Procion Turquoise MX-G if sodium sulfate, also known as Glauber's salt, is substituted for the regular salt (sodium chloride) ordinarily used in vat dyeing with fiber reactive dyes.
Now, if you use this dye for tie-dyeing, you may never add any salt at all. It is usual to skip the use of salt when applying the dye directly, as when tie-dyeing. The high concentration of the dye solutions applied makes the use of salt unnecessary. Sometimes people avoid the use of salt in low water immersion dyeing (LWI), whereas other times they add salt to increase the crystalline-like patterns.
However, even if you never add salt, neither table salt nor Glauber's salt, you may inadvertantly be adding salt in your dye powder. All dye powders must be diluted with some inert powder to adjust them to a standard dye strength per gram of dye. Sometimes sodium sulfate is used for this purpose; other times the synthetic tannin known as Tamol is used. (See What is in Procion MX dye powder?.) Colorado Wholesale Dye pride themselves on never using salt in dye; other companies may use varying amounts, possibly differing from one dyelot to another.
The effect may be that your turquoise will dye more intensely when its dye powder contains Glauber's salt. Other dye colors are unlikely to be affected. If you are dyeing with a pre-mixed dye color which contains turquoise and another dye, the presence of sodium sulfate might shift your resulting color more toward blue, away from green or purple. So, the use of different amounts of sodium sulfate may result in additional inconsistency in the color produced by turquoise MX-G. I do not know whether the effect is great enough to be noticeable in actual practice.
Every few years someone asks me for the chemical structure of the Procion 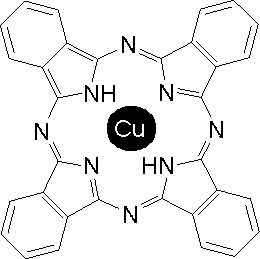 Turquoise MX-G dye molecule. This turns out to be more difficult to pin down than you might have imagined. I was unable to find it listed in the printed version of the Colour Index that I consulted.The chromophore of reactive blue 140 centers on the Copper Phthalocyanine molecule, which is a porphyrin ring with a copper atom in its center. (Click on the image to the right to see a larger version.) Copper phthalocyanine is the most beautiful turquoise chromophore, found in many different types of dye. Unfortunately, getting a description of how the other parts of the dye molecule attach to the copper phthalocyanine is complicated; in fact, there is not one structure, but instead a mixture of several closely related ones! The Giles Laboratory Course in Dyeing (a highly recommended book available internationally by mail-order from the Society of Dyers and Colourists for £9 plus shipping) gives its structure as shown below, on the left.
Turquoise MX-G dye molecule. This turns out to be more difficult to pin down than you might have imagined. I was unable to find it listed in the printed version of the Colour Index that I consulted.The chromophore of reactive blue 140 centers on the Copper Phthalocyanine molecule, which is a porphyrin ring with a copper atom in its center. (Click on the image to the right to see a larger version.) Copper phthalocyanine is the most beautiful turquoise chromophore, found in many different types of dye. Unfortunately, getting a description of how the other parts of the dye molecule attach to the copper phthalocyanine is complicated; in fact, there is not one structure, but instead a mixture of several closely related ones! The Giles Laboratory Course in Dyeing (a highly recommended book available internationally by mail-order from the Society of Dyers and Colourists for £9 plus shipping) gives its structure as shown below, on the left.
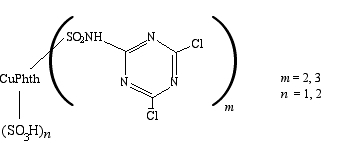 (The abbreviation "CuPhth" refers to the copper phthalocyanine, shown above on the right.) The m and n in the formula do not indicate a single structure. There may be one or two sulfonate groups on the phthalocyanine, there may be two or three dichlorotriazine sections per copper phthalocyanine, and the positions of these different items on the phthalocyanine ring are unknown and presumably random! (Thanks to Dr. François Denis for explaining this to me.)
(The abbreviation "CuPhth" refers to the copper phthalocyanine, shown above on the right.) The m and n in the formula do not indicate a single structure. There may be one or two sulfonate groups on the phthalocyanine, there may be two or three dichlorotriazine sections per copper phthalocyanine, and the positions of these different items on the phthalocyanine ring are unknown and presumably random! (Thanks to Dr. François Denis for explaining this to me.)
We usually know exactly what the colored dye molecule is in any of our Procion MX type dyes, there being only one in each unmixed single-hue dye, but turquoise MX-G is actually a combination of different dye molecules, even before the association of the turquoise dye molecules into dimers, trimers, etc., which occura after they have been mixed with water for a few days.
In conclusion, Procion Turquoise MX-G, reactive blue 140, is a beautiful dye that I use frequently, but it is not as predictable in use as other dyes of its class.
There are many other copper phtalocyanine dyes. For example, among fiber reactive dyes alone, we have the following:
One definitely non-phthalocyanine turquoise reactive dye is Lanaset Blue 5G (for use on wool, not cotton). One other fiber reactive turquoise dyes that might not be a phthalocyanine is Levafix Turquoise EG, but it, too, is likely to be a phthalocyanine. I once bought some Blue MX-3G, C.I. reactive blue 1, from Ajo Dyes, but they were in the process of discontinuing it at the time. There are many other non-copper phthalocyanine blue dyes, but all are much redder than the cyan-colored dyes that include copper phthalocyanine.
Non-fiber reactive dyes that are based on the copper phthalocyanine chromophore include Direct Blue 86 and the Ingrain dyes in which copper phthalocyanines are actually manufactured in the cotton fabric itself. For more information on all of these and other dyes, see the book, Giles's Laboratory Course in Dyeing Fourth Edition, by David G. Duff and Roy S. Sinclair. It is well worth ordering directly from the Society for Dyers and Colourists, even with overseas postage; used copies on Amazon.com, in the US, may cost ten times as much.
Advertisement
All of the pages on this site are copyright ©1998‑2026 Paula E. Burch, Ph.D.
Last updated: October 8, 2008
Page created: August 26, 2006
Downloaded: Friday, January 30, 2026