« 2011 March | Main | 2011 January »
Thursday, February 24, 2011
What does it mean when a commercial t-shirt company advertizes that they dye their shirts with wine, rum, chocolate, or Florida key limes?




Fabric paints are used for pigment dyeing


Natural Dyes Book
Use clear extender to
turn red clay into
a good fabric paint
(Please help support this web site. Thank you.)
Tuesday, February 22, 2011


Enameled canning pots make good dyeing kettles


Granite Ware 21-1/2-Quart Steel/Porcelain Canner


Country or region: California
Message: Hi,
My wool jersey, dyed with Jacquard acid dye at 185 F, has come out splotchy brown and black. Not heathered. Great areas of dark brown with black splotches. I'm going for black. Should I try a different dye or what? I washed with synthrapol before and after btw. Heat for 2 hrs, 185 for 35 minutes. Do not trust the acid dye enough to use again.
Wednesday, February 16, 2011




Tom Rolofson and Martine Purdy's DVD


Advanced Tie Dye Techniques: Making Shapes and Mandalas

Tuesday, February 15, 2011
How do I crackle dye a single color? I am removing color from four gauze dresses for my wedding....
Country or region: USA
Message: How do I crackle dye a single color? I am removing color from four gauze dresses for my wedding...never done this before...I just want pastel varigated. One lavender, one blue, one moss green, might try one with blue and purple and green. If I ruin them, I'm up a creek ! LOL I know, I'm nuts...I'm terrified....I love your work.
Monday, February 14, 2011
Procion H and other alternatives to H. Dupont silk painting dye
from a master


DVD: Silk Painting
With Jill Kennedy


Jacquard Green
Label:
Remazol dyes
for use on silk without steaming
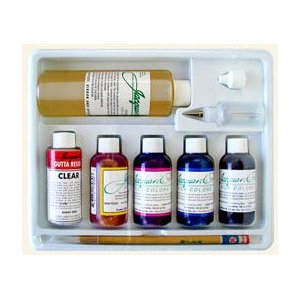

Jacquard Silk Color Kit dyes and instructions




Silk Painting: The Artist's Guide to Gutta and Wax Resist Techniques
by Susan Moyer


Country or region: South Africa
Friday, February 11, 2011
Problems in washing out Inkodye light-activated dye
Instructions for

 Artistic Photographic Processes
Artistic Photographic Processes
by Suda House

Country or region: USA
Message: I am using Inkodye to make photographic prints on cotton and leather using contact printing via negatives and I am having a problem with the 'washing out'.
Tuesday, February 08, 2011
Can you show me how to tie a particular pattern if I show it to you?
Sunday, February 06, 2011
I am looking for the molecular formula for Acid Yellow 19
Country or region: USA - Ohio
Message: I am looking for the molecular formula for PRO washfast acid yellow 119. Your site states that the C.I. is Acid Yellow 19, but I'm having difficulty locating either the CAS# or the molecular formula. Can you help? Thanks in advance.

Saturday, February 05, 2011
Can I dye a 100% polyester, micro-suede, fleece-lined, fur-collared coat?
 Country or region: North America
Country or region: North America
Message: Hello,
I have a 100% polyester, micro-suede, fleece-lined, fur-collared coat. My question is, can I dye this coat? I wanted to dye it a dark smoky blue.
Thursday, February 03, 2011
What kind of dyes do you recommend for dyeing pulp?
Country or region: US
Message: What kind of dyes do you recommend for dyeing pulp? We are currently using Rit Dye, but is very expensive.
Tuesday, February 01, 2011
I need the chemical structure of basic red dye, and whether azo dyes are considered basic, and what is the meaning of remazol dyes.


Linda Knutson's
Synthetic Dyes for Natural Fibers


The Chemistry and Application of Dyes
What does it mean when a commercial t-shirt company advertizes that they dye their shirts with wine, rum, chocolate, or Florida key limes?
Name: Mark
—ADVERTISEMENTS—
Fabric paints are used for pigment dyeing
Natural Dyes Book
The Art and Craft
of Natural Dyeing:
Traditional Recipes for Modern Use
by Jim Liles
Use clear extender to
turn red clay into
a good fabric paint
Country or region: Idaho, USA
Message: I just want to thank you for your post. You answered a question I had right on the money. I was looking at a crazy shirts catalog and wondered about what they were saying regarding their dyeing. The fact that you actually refered to them totaly told me what I wanted to know. Thank you for your time and effort to make this information available. Mark
Thanks for taking the time to let me know. It's good to know that someone is finding this material useful!
Mark is referring to an old post whose validity is still unchanged, in the archives from Thursday, September 14, 2006:
I'm hoping that you don't mind that I am emailing you with my question (I was searching websites and came across yours). I have seen t-shirts that have been dyed with coffee, different types of alcohol (beer, wine etc) and chocolate. Can we do this at home as well? The colours are very beautiful and was wondering if this is possible. Would the t-shirts be coloured to start with or would a natural dye made with say chocolate produce this result? I would love to hear back from you if you have the chance.
The problem with those shirts that you have seen is that they are kind of a scam. Note that when they say they are dyed "with" or "from" those natural substances, they do not specify that they used *only* those substances. My guess is that they may have added a tiny bit of the food or drink in question to either a dyebath of synthetic dyes, or, more likely, synthetic pigment "dyes" (which are not actually true dyes); then again, they may have omitted the foodstuff altogether.
You simply cannot dye cotton to be truly washfast with beer or wine or chocolate. You may be able to get a light stain, but not a reliable wash-proof dye. Unlike proper dyes, the coloring agents in these foodstuffs have little affinity for cotton, and will not stay. Coffee and tea can be used as dyes, but they are not permanent on cotton and will gradually fade, if you launder them. Most pigments found in foods cannot in themselves dye cotton at all well, though they can in theory be glued to them by using a binder such as is used in fabric paint. (You can dye cotton with grapes, if you mordant with alum and then with tannin and then with alum again, heat the cotton with the grapes repeatedly for several days, avoiding boiling since boiling will turn the grapes brown, but I would not advise you to wash a shirt dyed this way.)
Here's an example. The company Crazy Shirts Hawaii sells wine-colored t-shirts described as follows: "Like fine wine, this T improves with age. We color our soft 100%-cotton T with specialty dye made from red wine, and the result is deep, rich and full bodied." However, as famed tie-dyer Michael Fowler pointed out in a discussion of this subject on his old Tie-dyed.com forum, if you look closely at the picture, you can see from the color of the tag in the neck of the shirt on their site that this is a pigment-dyed shirt. Real [cotton] dyes will not "take" on the synthetic material of the tag, but the fabric paints known as pigment dyes will. The primary pigment in the shirts cannot be the anthocyanins that naturally color wine, because these chemicals are unstable when laundered (or even during the dyeing stage) and will end up turning brown, not improving with age by any means. It is pretty much certain that the "specialty dye made from red wine" contains a large proportion of wine-colored synthetic pigment. There's probably little difference between them and any other wine-colored pigment-dyed t-shirt from any other vendor.
Another example: "A tangy favorite! Our Key Lime dye dips into Florida Key Limes to get that tart citrus green. With it, we dye our creamy-soft 100%-cotton Crewnecks and Scoop Necks and add imaginiative CrazyShirts designs." Of course, ripe Key limes are not green at all; they are yellow. Furthermore, Key limes are no longer commercially produced in Florida; the Key limes we see for sale in the US come from Mexico. Unripe Key limes would be a terrible dye, because chlorophyll simply does not work at all well as a dye. Throughout history, green clothing and green tapestry yarn have been prepared by dyeing twice, once with the natural blue dye indigo, and then again with a yellow dye such as weld. Green plants were not used as a source of green dye, because the color produced is muddy and tends to fade or turn brown in the light. To add more humor to the claims about how these shirts were dyed, the "Key lime" shirts are a bluish green, certainly not the color of any lime, and not the color that a green plant produces if one insists on using it as a dye.
A practical issue concerning dying t-shirts with food is the sheer quantity of the foodstuff required. To dye fiber with a natural dye, you typically need to use at least an equal weight of natural dyestuff to fabric, somethings two or three times as much. Imagine - if it were possible to dye a t-shirt with chocolate, you'd need one to three pounds of chocolate for every pound of fabric! Even if the color of the chocolate were not just a stain that would wash out, would it be worth spending the money on that much chocolate, without even getting to eat any of it? Picking on Crazy Shirts Hawaii yet again: "The darkest, mellowest Rum is smooth as velvet - and that's what we use in our specialty dye for our Rum-Dyed Ts." Can you imagine using an entire quart of expensive dark rum to dye one t-shirt? Nobody does that! And a good thing, too, because the color produced could only disappoint you. Cotton is a fiber that is far more difficult to dye than wool; many natural dyes that work on wool are practically useless on cotton. Beer and rum are not going to produce exciting results even on wool, though.
In contrast, red dirt really can be used as a dye, though the only long-lasting color to result, iron buff, is a tan color, not the exciting bright red color of clay that inspires people to want to use it for dyeing. The easiest way to get a long-lasting bright color from red clay is to use it as a pigment and mix it with a clear acrylic binder manufactured for use in fabric paint, such as Versatex Clear Extender. A traditional Japanese alternative would be to use freshly made soy milk as a binder, though the results are less resistant to laundering; see Table Rock Llamas for one set of instructions.
You simply cannot dye cotton to be truly washfast with beer or wine or chocolate. You may be able to get a light stain, but not a reliable wash-proof dye. Unlike proper dyes, the coloring agents in these foodstuffs have little affinity for cotton, and will not stay. Coffee and tea can be used as dyes, but they are not permanent on cotton and will gradually fade, if you launder them. Most pigments found in foods cannot in themselves dye cotton at all well, though they can in theory be glued to them by using a binder such as is used in fabric paint. (You can dye cotton with grapes, if you mordant with alum and then with tannin and then with alum again, heat the cotton with the grapes repeatedly for several days, avoiding boiling since boiling will turn the grapes brown, but I would not advise you to wash a shirt dyed this way.)
Here's an example. The company Crazy Shirts Hawaii sells wine-colored t-shirts described as follows: "Like fine wine, this T improves with age. We color our soft 100%-cotton T with specialty dye made from red wine, and the result is deep, rich and full bodied." However, as famed tie-dyer Michael Fowler pointed out in a discussion of this subject on his old Tie-dyed.com forum, if you look closely at the picture, you can see from the color of the tag in the neck of the shirt on their site that this is a pigment-dyed shirt. Real [cotton] dyes will not "take" on the synthetic material of the tag, but the fabric paints known as pigment dyes will. The primary pigment in the shirts cannot be the anthocyanins that naturally color wine, because these chemicals are unstable when laundered (or even during the dyeing stage) and will end up turning brown, not improving with age by any means. It is pretty much certain that the "specialty dye made from red wine" contains a large proportion of wine-colored synthetic pigment. There's probably little difference between them and any other wine-colored pigment-dyed t-shirt from any other vendor.
Another example: "A tangy favorite! Our Key Lime dye dips into Florida Key Limes to get that tart citrus green. With it, we dye our creamy-soft 100%-cotton Crewnecks and Scoop Necks and add imaginiative CrazyShirts designs." Of course, ripe Key limes are not green at all; they are yellow. Furthermore, Key limes are no longer commercially produced in Florida; the Key limes we see for sale in the US come from Mexico. Unripe Key limes would be a terrible dye, because chlorophyll simply does not work at all well as a dye. Throughout history, green clothing and green tapestry yarn have been prepared by dyeing twice, once with the natural blue dye indigo, and then again with a yellow dye such as weld. Green plants were not used as a source of green dye, because the color produced is muddy and tends to fade or turn brown in the light. To add more humor to the claims about how these shirts were dyed, the "Key lime" shirts are a bluish green, certainly not the color of any lime, and not the color that a green plant produces if one insists on using it as a dye.
A practical issue concerning dying t-shirts with food is the sheer quantity of the foodstuff required. To dye fiber with a natural dye, you typically need to use at least an equal weight of natural dyestuff to fabric, somethings two or three times as much. Imagine - if it were possible to dye a t-shirt with chocolate, you'd need one to three pounds of chocolate for every pound of fabric! Even if the color of the chocolate were not just a stain that would wash out, would it be worth spending the money on that much chocolate, without even getting to eat any of it? Picking on Crazy Shirts Hawaii yet again: "The darkest, mellowest Rum is smooth as velvet - and that's what we use in our specialty dye for our Rum-Dyed Ts." Can you imagine using an entire quart of expensive dark rum to dye one t-shirt? Nobody does that! And a good thing, too, because the color produced could only disappoint you. Cotton is a fiber that is far more difficult to dye than wool; many natural dyes that work on wool are practically useless on cotton. Beer and rum are not going to produce exciting results even on wool, though.
In contrast, red dirt really can be used as a dye, though the only long-lasting color to result, iron buff, is a tan color, not the exciting bright red color of clay that inspires people to want to use it for dyeing. The easiest way to get a long-lasting bright color from red clay is to use it as a pigment and mix it with a clear acrylic binder manufactured for use in fabric paint, such as Versatex Clear Extender. A traditional Japanese alternative would be to use freshly made soy milk as a binder, though the results are less resistant to laundering; see Table Rock Llamas for one set of instructions.
Tuesday, February 22, 2011
Name: Lucy
—ADVERTISEMENTS—
Enameled canning pots make good dyeing kettles

Granite Ware 21-1/2-Quart Steel/Porcelain Canner
Country or region: California
Message: Hi,
My wool jersey, dyed with Jacquard acid dye at 185 F, has come out splotchy brown and black. Not heathered. Great areas of dark brown with black splotches. I'm going for black. Should I try a different dye or what? I washed with synthrapol before and after btw. Heat for 2 hrs, 185 for 35 minutes. Do not trust the acid dye enough to use again.
It's very possible that you simply did not use enough of the black dye. No matter what you're dyeing, or what type of dye you're using, it takes more dye to get a dark black than to get any other color. Using too little black will produce an off color, such as brown, purple, or dark green, depending on which dye you use.
Since your color is uneven, though, a more likely problem is that, unknowingly, you did not start with a perfectly clean garment. On garments that have been used, very often there are invisible stains that will repel dye, resulting in lighter splotches here and there. Even clean, new garments can show this problem, if the chemicals used in the manufacturing process were applied unevenly. If the stains are invisible, there's no way to tell that they are there until it's too late, and washing with Synthrapol, although helpful, does not always remove everything.
If you don't use a large enough cooking pot, you will always get uneven colors like this. If the sweater cannot move freely and easily in the pot of water plus dye, then some sections will not get as much dye as others, so they will end up a lighter color. It is often necessary to stir frequently when dyeing, to prevent some sections from failing to get their share of the dye (though you must beware of felting caused by excessive agitation). How big was the pot you used to do your dyeing in? I would not use a pot smaller than two or three gallons, minimum, for a large sweater.
When dyers want to produce uneven, variegated colors, they place a garment into a small container, too small to allow the garment to move freely, and add the dye to it without stirring. This is the basis of the method of multi-color dyeing known as low water immersion dyeing. Perhaps you inadvertently did the same thing.
The best garments to dye are those sold specifically for dyeing, with a label that prominently says PFD (for Prepared For Dyeing) or RTD (for Ready To Dye). Most commercial garments can be redyed safely, but you have to expect a certain failure rate, if the garment was not sold specifically for dyeing. Always prewash anything you want to dye (as you did), using the hottest water the garment can tolerate. After dyeing, let the wool cool in the dyebath, before rinsing it out.
You will probably do fine with the same dye again, if you use a larger pot, more water, and at least twice as much dye, and be careful to stir. Don't forget the vinegar, as your source of acid. However, if you'd like to try another dye, the very best black dye for wool is Lanaset Jet Black. See "Lanaset Dyes: A Range of Reactive and Acid Dyes for Protein Fibers". You will probably have to use mail-order to buy these dyes. There are several different companies from which you can order them, including PRO Chemical & Dye, Paradise Fibers, and Earth Guild. You will also need to buy a product called Albegal SET, to help the dye to make a smooth, solid color. Read the manufacturer's instructions before you place your order, to make sure that you get all of the ingredients that you will need. (See "Immersion Dyeing using Sabraset/Lanaset Dyes".)
If your dyeing pot is too small, the most inexpensive alternative is an enameled steel canning pot. You can buy a 21-quart enameled steel canning pot for about $20 from Amazon, though I'd encourage you to buy locally if you have a good source. Stainless steel cooking pots last longer, since they do not chip, but they are considerably more expensive. Don't buy a pot made of reactive metal, such as aluminum, for use in dyeing.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Wednesday, February 16, 2011
Name: Cindy
—ADVERTISEMENTS—
Tom Rolofson and Martine Purdy's DVD

Advanced Tie Dye Techniques: Making Shapes and Mandalas
Country or region: California
Message: I am looking for an alternative dye supplier (I have had some difficulty with Pro Chemical & Dyes) and am looking at Dharma's Procion MX dyes. Do you possibly have an updated comparison chart? I called Dharma and they actually refer people to your site! There are many colors I have purchased from Pro chem that are not represented on your current (2006) version. Thank you, thank you, thank you!!!
Most of the one hundred and fourteen Procion MX type dyes sold by PRO Chemical & Dye are proprietary mixtures, mixed in-house, whose formulas are a big secret. The same is true of Dharma Trading Company and of Jacquard Products, but their proprietary mixes are different ones. The availability of these in-house color mixtures is one of the factors that makes these three the most popular sources of Procion MX type dyes, as it's a convenience to be able to order special color mixtures, instead of just the dozen or so unmixed single-hue dyes that are available. A proprietary dye mixture is available from only one source; there is no equivalency between a proprietary mixture at one dye retailer and even the most similar-looking dye mixture at another dye retailer. There will always be some differences.
Each company also sells single-hue unmixed dyes, in their Procion MX type dye lines. These are the ones on my chart that are the same regardless of whether you buy them from one supplier or another. Different batches of these dyes may be more or less dense, older or fresher, or more or less dusty, but the dye molecules that produce the color are the same, regardless of source.
The two new unmixed single-hue dyes introduced by ProChem in recent years are boysenberry and grape. All of their other new Procion MX type dyes are proprietary mixtures, different from any color mixture available elsewhere.
ProChem's Grape is Colour Index reactive violet 14, and is also available from Dharma, Jacquard, and other sources. It's an extremely useful red violet, quite a pretty color, and good for mixing other colors. ProChem calls it Violet MX-GN, but that's a meaningless designation; the true MX code for this lovely dye is Violet MX-2R. (To understand the MX codes, see my page, "What do the letters and numbers in the code name for a Procion MX type dye mean?".)
ProChem's Boysenberry is pinker than Grape, purpler than Fuchsia. I like it very much, though not as much as Grape. Boysenberry is especially useful for mixing blood reds. My best guess is that its Colour Index name is reactive violet 13, but I can't be sure because the ProChem people told me they didn't know what its generic name is. It is certainly a single-hue, unmixed color, but as far as I know no other retailer is selling it yet under any name. ProChem calls their Boysenberry Violet MX-BR, which is no more meaningful than the made-up MX code they apply to violet. If it really is C.I. reactive violet 13, then its MX code should be Magenta MX-B.
A few of ProChem's pre-mixed Procion MX type dyes are what are called manufacturer's mixes. These mixtures are made in the factory, rather than by ProChem, and can be sold to multiple retailers. Unlike the proprietary mixtures, the manufacturers' mixtures are the same from one supplier to another, like the unmixed single-hue colors. The manufacturers' mixes I know of from ProChem are PRO Black 608, called Black MX-CWNA, which Dharma sells as New Black and Jacquard as Warm Black, and PRO Strongest Red 312, called red MX-GBA, which Dharma sells as Chinese Red. You can safely assume that all other dye mixtures you can buy from ProChem, the ones that are not manufacturer's mixes, are available from no other retailer.
The unmixed single hue dyes that ProChem sells, which you can find at most other retailers, as well, are just these few: Golden Yellow (Yellow MX-3RA), Sun Yellow (Yellow MX-8G), Tangerine (Yellow MX-GR), Lemon Yellow (Yellow MX-4G), Strong Orange (Orange MX-2R), Mixing Red (Red MX-5B), Fuchsia (Red MX-8B), Basic Blue (Blue MX-R) Mixing Blue (Blue MX-2G 125, which is 25% stronger than a standard strength of Blue MX-2G), Intense Blue (Blue MX-G), Turquoise (Turquoise MX-G) Deep Navy (Blue MX-4GD), and Burnt Orange (brown MX-GRN).
As you can see from the charts on my page, "Which Procion MX colors are pure, and which mixtures?", which has not been updated recently because its information is still correct, ProChem's Lemon Yellow is not sold by Dharma at all; however, ProChem's Sun Yellow, which is the most popular cool yellow, is available as Dharma's Lemon Yellow. ProChem's Golden Yellow (Yellow MX-3RA) is not available in quite the same form at Dharma, but the exact same dye with a different density is available as Dharma's Deep Yellow (Yellow MX-3R). ProChem's Burnt Orange (brown MX-GRN) is not sold by Dharma, but it is available from Jacquard as Rust Orange, assuming they have not replaced it with a mixture. ProChem's Deep Navy (Blue MX-4GD) is not available at Dharma, but the very similar Mixing Blue (Blue MX-2G) is available everywhere else as Cobalt Blue.
That leaves eleven single-hue pure dyes that are equally available at Dharma and at ProChem, and two more manufacturer's mixes that are the same between the two suppliers. For all of the other colors you've been ordering from ProChem, you will have to either find a substitute among a similar dye mixture from Dharma or Jacquard, or continue to order from ProChem. Since you are located in California, it does make sense to try to order from one of the California suppliers, for shipping that is both less expensive and faster. Note that you can mail-order dye directly from Jacquard Products in California, by phone, but only for package sizes of eight ounces or more per color. Their small two-thirds ounce jars are available only from other retailers that they supply with dye. Dharma and ProChem sell jars as small as two ounces per color.
For a much more detailed list of many different suppliers for dyes and the supplies to use with them, see my page, Sources for Dyeing Supplies Around the World. For equivalencies among the Procion MX type dyes sold by a number of different companies, see Which Procion MX colors are pure, and which mixtures?.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Tuesday, February 15, 2011
How do I crackle dye a single color? I am removing color from four gauze dresses for my wedding....
Country or region: USA
Message: How do I crackle dye a single color? I am removing color from four gauze dresses for my wedding...never done this before...I just want pastel varigated. One lavender, one blue, one moss green, might try one with blue and purple and green. If I ruin them, I'm up a creek ! LOL I know, I'm nuts...I'm terrified....I love your work.
Oh, dear. I can't recommend that you dye any commercial dress that you can't afford to ruin. What are you going to do if the results come out wrong? What if the dresses turn out to take up dye unevenly, with one panel of the fabric coming out a much darker color than any of the others? That's happened to me. What if there's a big splotch, currently invisible, of some sort of fabric finish, that prevents the dye from taking in that area? These problems are not uncommon in garments that are not specifically sold to be dyed. It's safer to buy garments labeled PFD (for Prepared For Dyeing) or RTD (for Ready To Dye).
Next problem: are your gauze dresses washable? You can't dye anything that isn't washable. Very few dresses that people buy to wear in weddings are washable.
Third problem: can you remove the color from the dresses as they are now? Some clothing is colored with dyes that cannot be removed, no matter what you do. It's impossible to tell whether or not this is true of your dresses until you try. What color are the dresses now, that is, what color are you trying to remove?
If the dresses are 100% cotton or linen, you can try ordinary chlorine-type household bleach, but you'd better not do this if they contain any spandex or nylon or silk. Chlorine bleach is death to synthetic fibers, except for viscose rayon. You can use a sulfur-based dye discharge chemical, such as Rit Color Remover, but only if your dresses can tolerate hot water. All sulfur-based color removers require heat, the hotter the better. It's easiest to try several boxes of Rit Color Remover in a washing machine with very hot tap water; if that doesn't work, sometimes the hotter temperatures you can reach in a cooking pot on the stovetop work better, but it's much less convenient. See "What chemicals can be used to remove dye?"
Fourth problem: if everything else has gone well up to this point, and you've washed the dresses and successfully removed their color - if all of that turns out to be possible and to have worked well for you - then you can ask how to crackle dye them with one color. It's not difficult to do, but you must know what the fiber content of the dresses is, so that you can choose the right kind of dye. Cotton cannot be dyed with dyes that work on polyester, and wool requires completely different dyes than either. Some fiber blends, such as poly/spandex, simply cannot be dyed, because the high heat required to dye polyester will destroy the spandex. Crackle dyeing is done, using whatever type of dye is required for your fiber, according to the instructions on my page of How to Do Low Water Immersion Dyeing. However, it is very important to choose a dye that contains only one color of dye molecule, because dyes that are premixed from more than one color will separate out into multiple colors when you do the crackle dyeing. The results can be beautiful, but it doesn't sound like what you want at all. This means that you need to find a single-hue, unmixed dye color to use, for each color you want. If you get to this point, email me with the exact fiber content of your dresses, and I might be able to help you find appropriate dye choices.
Alternatively, if the dye in the dresses is easily removed, you could do a sort of crackle dyeing in reverse, by cramming each dress into a small cooking pot before applying Rit Color Remover. This would require a serious amount of trial and error first, however. You cannot expect to get good results without extensive testing. The Rit Color Remover or other dye removal chemical might work as desired, turning the original color into white, wherever it can reach the fabric, but it's just as likely to turn the colors to some new and unexpected color, possibly one that you think is ugly. Green can turn orange, violet can turn yellow, black can turn brown; it's unpredictable, since you don't know what dyes have been used already in your dresses.
Good luck. Although each of the things you want to do can be easy and fun and rewarding, being required to have it come out perfectly the very first time you try it makes the whole project nearly impossible.
Another way to get the dresses you want would be to buy some PFD dresses that are made of cotton or rayon, and dye them (the best site for clothing blanks is Dharma Trading Company, but they don't carry formal attire), or to dye some PFD fabric and dye it, or buy some fabric whose colors and patterns you like already, and get a local seamstress to sew them into dresses for you. This last would probably be the wisest choice.
(Please help support this web site. Thank you.)
Monday, February 14, 2011
Procion H and other alternatives to H. Dupont silk painting dye
Name: Juliet
—ADVERTISEMENTS—
Learn silkpaintingfrom a master

DVD: Silk Painting
With Jill Kennedy
Jacquard Green
Label:
Remazol dyes
for use on silk without steaming

Jacquard Silk Color Kit dyes and instructions

Silk Painting: The Artist's Guide to Gutta and Wax Resist Techniques
by Susan Moyer
Country or region: South Africa
Message: Thanks for your excellent site. I have a question about Procion H dyes. I read somewhere (can't remember where, i think on your site) that it cannot cross the barrier into the cells of the body. Is this correct? I have had bad reactions to Dupont silk painting dyes and acid dyes and am hoping the H will work for me. I see Jacquard is discontinuing the Liquid H range. They are not responding to my query as to why this is. Do you know the answer? Also read on the Jacquard blog that Procion H requires only steaming and no chemical water etc - did I understand that correctly? Maiwa is now the only site that I can find ....anywhere...that carries liquid Procion H dyes ? Tx and kind regards
I wonder if your reaction to the Dupont dyes was caused by the solvents they contain. The biggest problem with the Dupont silk painting dyes, as with other brands of French silk dyes, is that they are prepared with solvents that produce harmful fumes. Their use therefore requires good ventilation, which is unfortunately in short supply in many art studios. Wearing a dust mask will not help; in fact, not even a cartridge respirator can make it safe to breathe fumes from dye mixtures that contain alcohols, as the H Dupont dyes do. Open windows or fume extraction systems are required in order to work safely with solvents. I wrote about these dyes in a Dye Forum post entitled, "What's in the French silk dyes?".
Some of the French silk dyes contain basic (or cationic) dyes, some contain acid dyes, and a few even contain fiber reactive dyes. Cationic dyes penetrate inside cells and cell nuclei more readily than negatively charged dyes, according to the US National Library of Medicine's Toxnet Toxicology Data Network. In contrast, fiber reactive dyes, as well as most of the dyes sold as acid dyes, are negatively charged. (Some basic dyes, such as Rhodamine B, are sold in several lines of acid dyes.) Fiber reactive dyes tend also to react with the proteins on the surface of the dead skin cells that protect underlying layers of skin, rather than penetrating inside living cells, which makes them useful for distinguishing between living and dead cells in scientific studies; this is reassuring for us, but it is still wise to wear gloves and avoid skin contact with all textile dyes.
Fiber reactive dyes make an excellent alternative to the solvent-containing silk dyes for silk painting. You can work with fiber reactive dyes that have been dissolved in water instead of more dangerous solvents; the chemicals used with fiber reactive dyes do not have the safety problems of the solvents used in the French silk dyes. There are several different classes of fiber reactive dyes that can be used as silk dyes, including Procion MX, Procion H, Drimarene K, and Remazol (vinyl sulfone) dyes.
Procion MX dyes can be used for silk painting, but their rapid reactions either with the fiber or with water means that you cannot keep colors in liquid form for more than a couple of weeks. The color balance in your mixtures may shift as the fastest-to-react color goes bad. The dyes work well on silk, but you are likely to find it inconvenient to mix up your colors anew every week or two.
Procion H dyes are extremely similar to Procion MX dyes, but much less reactive; they require steaming to be fixed on the silk, and Procion H dyes that you have dissolved in water will stay good for a long time. Jacquard Products has discontinued their Procion H liquid dyes in favor of another fiber reactive dye, but you can still buy liquid Procion H dye mixtures from PRO Chemical & Dye in the US and from G&S Dye in Canada. However, since you live in South Africa, shipping of liquid dyes seems likely to be prohibitively expensive.
Remazol or vinyl sulfone dyes should work as well for you as Procion H dyes. (See "About Vinyl Sulfone Fiber Reactive Dyes".) This is the line of dyes that Jacquard Products has substituted for their old line of Procion H liquid dyes. They expect the Liquid Remazol dyes to be more popular, because they work as well, but are more concentrated and therefore less expensive. Like Procion H dyes, the Remazol dyes are fiber reactives. They are less reactive than Procion MX dyes, so you can mix up a specific color of dissolved dye and expect it to stay good for several months, especially if you store your stock solutions in the refrigerator. (Before using refrigerated dyes, allow them to return to room temperature, and shake well to dissolve any dye that has settled out.) They can be set on silk at warm room temperatures using high-pH chemicals such as washing soda, or, for silk painting, they can be used without auxiliary chemicals, or mixed with a sodium alginate thickener for a different paint texture, and then steam-set. An advantage of the fiber reactive dyes, including both Remazol and Procion H, is that they require only thirty minutes of steaming, instead of the long steaming of up to three hours that is required for the French silk dyes.
Remazol dyes can be purchased from a number of different sources in many different countries. Some sources sell only the powdered form, but you can dissolve the dyes yourself safely. Dye powders are allergenic if you breathe them, but you can work with them safely by wearing a dust mask when measuring them out. Once you've mixed them with water, no dust mask is needed, only the usual protection of thin disposable waterproof gloves made of latex or nitrile. Jacquard Products' line of liquid Remazol type dyes is called Vinyl Sulphon, while PRO Chemical & Dye calls them Liquid Reactive Dyes. You can also buy vinyl sulfone dyes under other brand names. Most convenient for you would be to buy Slipstream dyes, which are vinyl sulfone dyes in powder form that are available to hand dyers in South Africa. See Slipstream Dyes and Prints, or contact Melanie Brummer in Johannesburg by calling 0835689150 or sending e-mail to info [at] dyeandprints.co.za. You can mail-order the dyes, or ask for the name of a local stockist who sells them.
Please let me know if you obtain powdered vinyl sulfone dyes and need help with the recipes for working with them for painting silk. Instructions for dyeing with Remazol and Procion H type dyes usually call for chemical water, but the dyes can fix on silk when steamed, even without chemical auxiliaries. Baking soda is a good chemical auxiliary, because it is converted to the higher-pH soda ash only when subjected to high heat when the silk is steamed. If you have hard water, I strongly recommend the use of the water softener sodium hexametaphosphate in your dye mixtures, or use softened, distilled, or deionized water.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Friday, February 11, 2011
Problems in washing out Inkodye light-activated dye
Name: Jessica
—ADVERTISEMENT—
Instructions for
using Inkdoyes

by Suda House
More books about photos on fabric
Country or region: USA
Message: I am using Inkodye to make photographic prints on cotton and leather using contact printing via negatives and I am having a problem with the 'washing out'.
I cannot get the 'unexposed' areas of the print to effectively 'wash out' - they keep 'developing' and thus my beautiful photographic print disappears over time. I understand that boiling water should help, but I'm wondering if there is some chemical that I could apply to the print that would effectively 'stop' the reaction, 'stop' the oxidation of the leuco vat dye so that my print stays looking great!
I'm trying to complete a project very dear to my heart, please help me find a solution. Thank you very much!
I'm trying to complete a project very dear to my heart, please help me find a solution. Thank you very much!
Are you using cold running water for your developing stage, changing the water very frequently? And are you then following this with warm soapy water?
The best book for instructions in using Inkodyes is Suda House's book, Artistic Photographic Processes. She goes into far more detail, useful detail, than any other source I've seen. After applying the dye to the surface, letting it dry, and exposing it with the masks to an ultraviolet light source, she says,
"7. Before development in ordinary room light, prepare two trays, one for cold running water and the other filled with warm, soapy water. (Suggestion: Dilute 1 tablespoon [15 ml] of liquid soap in 1000 cc [one liter] tap water.) Develop by immersing the fabric in the tray of cold running water for 5 minutes. The dye from the unexposed areas will stain your hands, so rubber gloves are advisable. Change the water in the tray every minute, so that excess dyes run off and do not contaminate highlight or unexposed areas. Next, place the fabric in the tray of warm, soapy water, continuously agitating the tray for 5 minutes.
"8. The fabric may be vigorously rubbed in the soapy water; this is sometimes necessary to clear the highlights from possible staining or overexposure. Some photographers actually develop large fabric pieces in the washing machine, first in a 10-minute agitation cycle without soap and then in a cycle with soap added. Complete with two rinse cycles
"9. At this point, if the dyes are still running, repeat step 8 until no more dye colors the water. Development is now complete."
"8. The fabric may be vigorously rubbed in the soapy water; this is sometimes necessary to clear the highlights from possible staining or overexposure. Some photographers actually develop large fabric pieces in the washing machine, first in a 10-minute agitation cycle without soap and then in a cycle with soap added. Complete with two rinse cycles
"9. At this point, if the dyes are still running, repeat step 8 until no more dye colors the water. Development is now complete."
Following those steps, the image is to be allowed to dry flat on blotter paper, and then ironed at the cotton setting on the wrong side, in order to complete the permanent setting of the dyes. Other sources recommend baking, instead of ironing.
The cold running water development may be performed under ordinary room light, she says, but the preceding steps are done under a yellow safe light, one which presumably emits no ultraviolet light. You're probably being careful not to use room light that emits a large amount of ultraviolet during the washing-out process, but if not, that's another thing to consider. (I believe that fluorescent lights emit more ultraviolet than incandescent lights do.)
The Inkodye information on my site is found in two places: "About Vat Dyes" and "How to Dye and Paint Fabric with Light". Neither of these pages goes into nearly the same detail as Artistic Photographic Processes. The book is out of print, having been published in 1985, but used copies are readily available for only a few dollars.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Tuesday, February 08, 2011
Can you show me how to tie a particular pattern if I show it to you?
Name: Jeff
Country or region: New Jersey
Message: Can you show me how to tie a particular pattern if I show it to you?
Sometimes I can, sometimes I can't. What you should do, instead, is join the Dye Forum Community of Dyers on my site, and post your question to the group there. That way you can get input from me and potentially from a great many other dyers. It's best if you include a picture.
There's no fee to join the Dye Forum, and no email except for the initial account set-up. You do have to reply to the automatic email you get when you sign up, in order to prove it's not another of the dozens of robospammer sign-ups I get every day.
The Dye Forum Community of Dyers is located here:
Click on the "Create new account" link in the right margin of the page, then reply to the email you receive with some sort of description of your dye-related interests.
The best place on the Dye Forum to post your query on how to tie a particular pattern would be in the Tie dyeing discussion.
The best place on the Dye Forum to post your query on how to tie a particular pattern would be in the Tie dyeing discussion.
Sunday, February 06, 2011
I am looking for the molecular formula for Acid Yellow 19
Name: Kimber
Country or region: USA - Ohio
Message: I am looking for the molecular formula for PRO washfast acid yellow 119. Your site states that the C.I. is Acid Yellow 19, but I'm having difficulty locating either the CAS# or the molecular formula. Can you help? Thanks in advance.
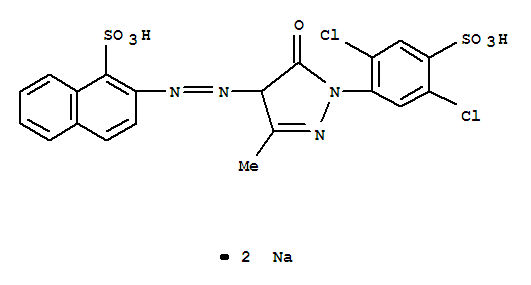
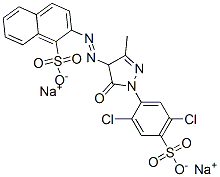
It looks like Colour Index Acid Yellow 19 is CAS No. 12220-64-3. The trick in searching is the careful use of quotation marks to group your phrases. I did a search with double quotes around "acid yellow 19" plus "CAS", and poked around a bit until I found a CAS number that other sites identified as having the right Colour Index name.
The molecular formula is given as C20H12Cl2N4Na2O7S2, and the IUPAC name as disodium 2-{2-[1-(2,5-dichloro-4-sulfonatophenyl)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-yl]diazen-1-yl}naphthalene-1-sulfonate. Here's an image from LookChem.com's Acid Yellow 19 page:

Here's a nicer drawing from ChemDatas.com, without the slightly confusing "Me" for that methyl group (which is not part of the way I'm used to drawing molecular structures), albeit with a perfectly reasonable watermark:
Chemicalbook.com has another nice drawing:

I see from searching for the CAS number that Dharma Trading Company has an MSDS for acid yellow 19 on their site, as evidently it's included among their new line of Dharma Acid Dyes. That would have been another good place to look. The same dye is included in the Jacquard Acid Dye collection as their #602 Bright Yellow. The MSDS for Dharma's brand of acid yellow 19 has the CAS number listed on it, but the MSDS pages for the Jacquard brand and the ProChem Washfast dyes don't include CAS numbers.
(Please help support this web site. Thank you.)
Saturday, February 05, 2011
Can I dye a 100% polyester, micro-suede, fleece-lined, fur-collared coat?
Name: Hannah
 Country or region: North America
Country or region: North AmericaMessage: Hello,
I have a 100% polyester, micro-suede, fleece-lined, fur-collared coat. My question is, can I dye this coat? I wanted to dye it a dark smoky blue.
Unlike many garments people ask me about, that one is machine washable, and it does not appear to be treated to make it water-resistant. As long as it has not been treated to make it stain-resistant, either, it's a good bet for dyeing.
You can't ever use any ordinary type of dye to dye polyester. All-purpose dyes such as Rit, fiber reactive dyes for cotton, and acid dyes for wool - all of these other types of dye will just wash out. You need to use a special kind of dye called disperse dye, which was developed for use on synthetic fibers.
The problem with dyeing polyester is that it requires high temperatures. Normally, you need to use at least half an hour in boiling water, not hot tap water, to get the disperse dye into the polyester fiber. Unfortunately, it's very unlikely that you have, or want to invest in, a cooking pot large enough to put a long fake fur coat into, with enough room for it to move freely! There has to be enough room for the coat to move freely in order for the dye to produce a solid color. If you stuff the coat into a pot that barely holds it, you will get more of a tie-dyed effect. I can't imagine a pot of less than five gallons being big enough for getting a solid color on your coat. You might possibly be able to call your local hand-weaving or spinning guild to ask whether there is anyone willing to lend or rent out a large dyeing kettle, but you won't want to spend the hundreds of dollars a new pot of that size would cost, until you are planning to make a habit of dyeing bulky items.
This brings us to a new product I saw listed for the first time only yesterday. Dharma Trading Company is selling new "industrial" disperse dyes. They write:
"These are what is known as a 'low energy' dye for Polyester, so they don't require the super high temperatures that the stuff is dyed with commercially, but the hotter the dye bath, the better/darker color you will get. The minimum recommended temperature is 160°F, and 190° does even better. The water soluble bags get thrown into a HOT water washing machine load along with the appropriate amount of liquid Carrier for 30 minutes and then rinsed and washed. "
They add, "The results are not as long-term, or as bright as commercial Polyester dyes, but these are easier to use, don't need as hot of water and are much less expensive. No fancy dyeing equipment needed, just a washing machine and access to hot water."
Now, nobody I know has a washing machine that will reach 160°F. The hottest normal tap water setting is 140°F, and most people set theirs lower, to 120°F, to reduce the risk that a child will be injured by scalding hot water in the sink or bathtub. It is possible, however, to temporarily raise the setting on your water heater (with all due care), and/or boil water on your stovetop in a large pot, and pour it into your washing machine, adding more hot water until the temperature of the water reaches over 160°F (better get it up above 170°F, since it will cool off quickly).
Since it takes some time for the dye to penetrate the fiber, you must stop the washing machine from draining when it reaches the end of its cycle, and reset it back to the beginning of the cycle, repeatedly, in order to allow enough time for dyeing. See my page, "How can I dye clothing or fabric in the washing machine?".
It is important to note that modern energy-saving washing machines are designed so that cold water mixes in to the hot water, when you set the machine to wash with hot water, so what you end up with is no more than warm, around 100 or 110°F at best. To get around this, when really hot tap water is truly needed, I turn off the faucet for the cold water supply that leads to my washing machine (it has to be turned back on before any rinsing will occur, since my washing machine does only cold water rinses). The water does cool some in the pipes, so use a thermometer to check the temperature of the water as it fills the machine.
I should warn you that some people have said that there might be some risk that very hot water will wear out any rubber or plastic hoses or other parts more quickly than water that is at or below the 140°F maximum that washing machines in the US are designed to handle.
My testing of another brand of disperse dye, Jacquard iDye Poly (which could possibly be the identical dye in a different packaging, or just a similar dye), showed that the blue color worked well on nylon and especially acetate at below a boil, but extensive heating at a full boil was required for full color on polyester. That won't be possible in a washing machine. I will be very curious as to how satisfactory you, or anyone else, finds this dye to be when used in a washing machine, with or without added hot water. I predict that it will be pretty useless without the addition of very hot water, since merely hot tap water is simply not hot enough to dye polyester.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, February 03, 2011
What kind of dyes do you recommend for dyeing pulp?
Name: Kim
Country or region: US
Message: What kind of dyes do you recommend for dyeing pulp? We are currently using Rit Dye, but is very expensive.
If Rit dye is working for you, but costs too much, then there's a ready solution. You can buy the same dye for plant fibers that's included in the Rit dye mixtures, for a lower cost than any other type of dye. The tiny packages or bottles of Rit dye are extremely expensive, simply because they contain mostly salt and detergent, but very little dye. If you buy direct dye in bulk, you can save much more than half of the money that' you've been spending on Rit dye.
Rit dye is a type of dye called an all-purpose or union dye (see "About All Purpose Dyes"), composed of two types of dye mixed together: a dye called direct dye that works on cotton and on other plant fibers such as paper pulp, and a dye called acid dye that works on animal fibers such as wool. Direct dye (see "About Direct Dyes") is the type of dye that is traditionally used for dyeing paper pulp. It is the least expensive of the types of dyes available. It's ironic that the form in which most people meet it, Rit dye, is one of the most expensive of all dyes, per pound of material dyed. You might be interested in seeing a chart I made, "Comparison of dye costs", which you can see in the Dye Forum community of dyers.
A good recipe for dyeing paper pulp with direct dyes can be found at the website of PRO Chemical and Dye. See their "Coloring Paper Pulp using Diazol Direct Dyes". Unfortunately, PRO Chemical & Dye no longer sells direct dyes, but there are other sources. (See my page listing "Sources for Dyeing Supplies Around the World".) Look at this page to see whether you can still buy Diazol Direct dyes at clearance prices from ProChem: "Clearance Diazol Direct Dyes!" It looks as though they still have quite a few colors.
Note that the packaged direct dyes are highly concentrated. Their package sizes are 8 ounces or one pound of direct dye. You need only 5 grams (one teaspoon) of direct dye powder to color one pound of paper pulp to a medium shade (less for paler shades, more for very intense or dark colors). One pound of Diazol direct dye, or any other industrial-strength direct dye, will color 90 pounds of paper pulp. One box of Rit dye contains no more than about 5 grams of direct dye powder! One bottle of Rit liquid dye is equivalent to only twice this.
The most economical source of direct dyes may be Dharma Trading Company, whose line of "Industrial dyes" is direct dye. You can buy one pound of direct dye powder for $6.89 (plus shipping), that will contain the equivalent of at least 90 boxes of Rit Dye, which would cost you closer to $200 to buy.
Another good mail-order source of dyes, in the US, is Aljo Mfg., in New York. They sell direct dyes similar to the Diazol dyes formerly sold by ProChem. Their line of direct dyes is called Aljo® Cotton & Rayon Dyes. You can see their dye selection on their website, but to order you must call them on the phone.
There are other brands of direct dye on the craft market, under the names iDye, Deka L, and Cushing Direct, but their prices are closer to those for Rit dye than to the other sources I've already listed. On the other hand, if you are willing to buy very large quantities of dyes, ten pounds or more of each color, then you can find even better prices per pound by buying in bulk from an industrial supplier such as Standard Dyes, Keystone Aniline, or Dystar.
When you buy your direct dye, you will probably also want to buy the cationic dye fixative, Retayne, or another brand of this sort of product, to fix the direct dye in place. Dharma and ProChem sell this; most dye suppliers do. See my page, "Commercial Dye Fixatives", for more information about this dye fixative.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Tuesday, February 01, 2011
I need the chemical structure of basic red dye, and whether azo dyes are considered basic, and what is the meaning of remazol dyes.
Name: Sobia
—ADVERTISEMENTS—
Books about the Chemistry of Dyes

Linda Knutson's
Synthetic Dyes for Natural Fibers
by Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
The Chemistry and Application of Dyes
edited by David R. Waring
and Geoffrey Hallas
Country or region: Pakistan
Message: I need the chemical structure of basic red dye, and whether azo dyes are considered basic, and what is the meaning of remazol dyes.
Remazol dyes are a type of fiber reactive dye. They can be used on cellulose fibers such as cotton, and they can also be used as true reactive dyes on wool, under completely different conditions. They are popular for both hand dyers and for industrial use; in the EU, many Remazol dyes have been approved even for use in baby clothes. For more information on Remazol dyes, see my page, "About Vinyl Sulfone Fiber Reactive Dyes".
Azo dyes include any dye that contain an azo group, which is a double-bonded double-nitrogen unit (-N=N-) in the chromophore (the colored part of the dye's molecular structure). They are formed by azo coupling, between a diazonium compound and an aromatic compound. The azo groups help to produce brilliant colors. If you look at the chemical structures of synthetic dyes in many different classes, you will see azo groups. For example, if you look at the structures of the Procion MX type fiber reactive dyes on my page, "What is the chemical structure of Procion MX dye?", you will see the -N=N- linkage in each chromophore. The same is true of many different classes of dyes.
However, azoic dyes, which, confusingly, some people may refer to "azo dyes", are a specific class of dye, also known as naphthol dyes. Many dyes that contain an azo group are not in the class of azoic dyes. For more information on naphthol/azoic dyes, see my page, "About Naphthol Dyes". There are more serious safety concerns with these than with most other classes of dyes. Some of the carcinogenic, mutagenic, or teratogenic members of this group of dye components can pass right through unbroken skin, permanently damaging the dyer, so more than ordinary precautions must be taken when working with this class of dyes. Cheap latex gloves that frequently develop holes are not adequate for working with naphthol dyes. As a general rule, I recommend against the use of this class of dyes by hand dyers, because of safety concerns.
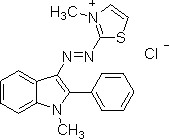
The chemical structure of your basic red dye depends on which basic red dye you are concerned with. The only thing you know for sure, from the fact that it's a basic (or cationic) dye, is that it carries a positive charge, unlike all other classes of dye. The naming convention for dyes listed in the Colour Index stars with the specific class of dye (e.g., "basic"), followed by a color base name (e.g., "red"), and finally a number; for example: "Colour Index Basic Red 1," often shortened to "basic red 1", or "Colour Index Basic Red 29," shortened to "basic red 29". We can have no idea which dye structure you want, unless you specify the full Color Index name, including that final number. There are dozens of different basic red dyes. See, for an example, the list of dyes currently available at Classic Dyestuffs, in North Carolina; if you do a search with "basic red" in the box, they list different brands of basic red 13, basic red 15, basic red 18, basic red 22, basic red 27, basic red 29, basic red 45, basic red 46, basic red 49, basic red 51, basic red 52, basic red 59, basic red 73, basic red 81, and basic red 104. Here is an image of the chemical structure of Basic Red 1, also known as Rhodamine 6, from the Chemical Book website:

(from http://www.chemicalbook.com/StructureFile/ChemBookStructure1/GIF/CB2674254.gif)
It does not appear to contain an azo linkage. The positive charge is not shown in the drawing, as such, but must be there.
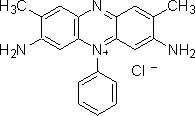
Here is an image of basic red 29, from Sigma Aldritch:

(from http://www.sigmaaldrich.com/thumb/structureimages/37/mfcd00013337.gif)
As you can see, it does contain an azo linkage, and a positive charge, so it is both an azo dye and a basic (cationic) dye.
Here is a structure for basic red 2, from Sigma Aldritch:

(from http://www.sigmaaldrich.com/thumb/structureimages/59/mfcd00011759.gif ).
As you can see, although this is a basic dye, as shown by its positive charge, it does not contain an azo dye linkage. There is no particular connection between a dye's belonging in the class of basic dyes, and whether or not a dye contains an azo group.
All basic dyes, which are also known as cationic dyes, have a positive charge on the dye molecule, unlike other classes of dyes. (Acid dyes and direct dyes have a negative charge, while vat dyes have a neutral charge.) Basic dyes are no longer considered useful for natural fibers, on which they have very serious lightfastness problems, but they are valuable in dyeing acrylic fibers, especially more intense colors are desired, since disperse dyes, which also work well on acrylic, do not produce deep shades on acrylic. For more information about hand dyeing with basic dyes, see my page, "Dyeing Acrylic with Basic Dye".
(Please help supportthis web site. Thank you.)
(Please help supportthis web site. Thank you.)



