I need to know the chemical structure of basic red dye, and whether azo dyes are considered basic, and what is the meaning of remazol dyes.
Name: Sobia
—ADVERTISEMENTS—
Books about the Chemistry of Dyes

Linda Knutson's
Synthetic Dyes for Natural Fibers
by Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
The Chemistry and Application of Dyes
edited by David R. Waring
and Geoffrey Hallas
Country or region: Pakistan
Message: I need the chemical structure of basic red dye, and whether azo dyes are considered basic, and what is the meaning of remazol dyes.
Remazol dyes are a type of fiber reactive dye. They can be used on cellulose fibers such as cotton, and they can also be used as true reactive dyes on wool, under completely different conditions. They are popular for both hand dyers and for industrial use; in the EU, many Remazol dyes have been approved even for use in baby clothes. For more information on Remazol dyes, see my page, "About Vinyl Sulfone Fiber Reactive Dyes".
Azo dyes include any dye that contain an azo group, which is a double-bonded double-nitrogen unit (-N=N-) in the chromophore (the colored part of the dye's molecular structure). They are formed by azo coupling, between a diazonium compound and an aromatic compound. The azo groups help to produce brilliant colors. If you look at the chemical structures of synthetic dyes in many different classes, you will see azo groups. For example, if you look at the structures of the Procion MX type fiber reactive dyes on my page, "What is the chemical structure of Procion MX dye?", you will see the -N=N- linkage in each chromophore. The same is true of many different classes of dyes.
However, azoic dyes, which, confusingly, some people may refer to "azo dyes", are a specific class of dye, also known as naphthol dyes. Many dyes that contain an azo group are not in the class of azoic dyes. For more information on naphthol/azoic dyes, see my page, "About Naphthol Dyes". There are more serious safety concerns with these than with most other classes of dyes. Some of the carcinogenic, mutagenic, or teratogenic members of this group of dye components can pass right through unbroken skin, permanently damaging the dyer, so more than ordinary precautions must be taken when working with this class of dyes. Cheap latex gloves that frequently develop holes are not adequate for working with naphthol dyes. As a general rule, I recommend against the use of this class of dyes by hand dyers, because of safety concerns.
The chemical structure of your basic red dye depends on which basic red dye you are concerned with. The only thing you know for sure, from the fact that it's a basic (or cationic) dye, is that it carries a positive charge, unlike all other classes of dye. The naming convention for dyes listed in the Colour Index stars with the specific class of dye (e.g., "basic"), followed by a color base name (e.g., "red"), and finally a number; for example: "Colour Index Basic Red 1," often shortened to "basic red 1", or "Colour Index Basic Red 29," shortened to "basic red 29". We can have no idea which dye structure you want, unless you specify the full Color Index name, including that final number. There are dozens of different basic red dyes. See, for an example, the list of dyes currently available at Classic Dyestuffs, in North Carolina; if you do a search with "basic red" in the box, they list different brands of basic red 13, basic red 15, basic red 18, basic red 22, basic red 27, basic red 29, basic red 45, basic red 46, basic red 49, basic red 51, basic red 52, basic red 59, basic red 73, basic red 81, and basic red 104. Here is an image of the chemical structure of Basic Red 1, also known as Rhodamine 6, from the Chemical Book website:
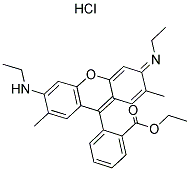
(from http://www.chemicalbook.com/StructureFile/ChemBookStructure1/GIF/CB2674254.gif)
It does not appear to contain an azo linkage. The positive charge is not shown in the drawing, as such, but must be there.
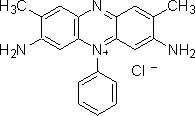
Here is an image of basic red 29, from Sigma Aldritch:
(from http://www.sigmaaldrich.com/thumb/structureimages/37/mfcd00013337.gif)
As you can see, it does contain an azo linkage, and a positive charge, so it is both an azo dye and a basic (cationic) dye.
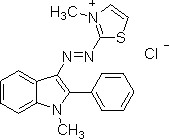
Here is a structure for basic red 2, from Sigma Aldritch:
(from http://www.sigmaaldrich.com/thumb/structureimages/59/mfcd00011759.gif ).
As you can see, although this is a basic dye, as shown by its positive charge, it does not contain an azo dye linkage. There is no particular connection between a dye's belonging in the class of basic dyes, and whether or not a dye contains an azo group.
All basic dyes, which are also known as cationic dyes, have a positive charge on the dye molecule, unlike other classes of dyes. (Acid dyes and direct dyes have a negative charge, while vat dyes have a neutral charge.) Basic dyes are no longer considered useful for natural fibers, on which they have very serious lightfastness problems, but they are valuable in dyeing acrylic fibers, especially more intense colors are desired, since disperse dyes, which also work well on acrylic, do not produce deep shades on acrylic. For more information about hand dyeing with basic dyes, see my page, "Dyeing Acrylic with Basic Dye".
(Please help supportthis web site. Thank you.)
(Please help supportthis web site. Thank you.)
Posted: Tuesday - February 01, 2011 at 07:25 AM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:49 PM
Total entries in this category:
Published On: Aug 29, 2012 02:49 PM

