« 2011 February | Main | 2010 December »
Monday, January 31, 2011
How can I dye faded car mats back to their original color?
Country or region: Australia
Message: Hello, I have some original OEM car mats out of a car I am restoring. They have faded/worn to a bluish colour. I don't know what material they are made of, but they are a thick almost shag pile carpet backed with rubber. They are a valuable item and no longer available so I want to restore them to black. I have tried using iDye Poly (black), but this was unsuccessful. This was most likely due to the fact I was unable to get enough heat into the process. The mats are heavy and large, so I can't just put them in a pot on the stove. I used hot tap water and let sit over night, but the final result was poor. Would you be able to recommend a dye/process that might allow me to return these mats to black?
Friday, January 28, 2011
My question is how to dye silk cocoons without heat, as it softens them too much
My question is how to dye silk cocoons without heat, as it softens them too much. I am doing a very large hanging of over 2000 and have already removed the worm. Each cocoon is in excellent condition and I want to keep them that way.
I have tested a few with dynaflow --I quickly dyed them, put them in a salad spinner and then under a heat lamp--this all worked great but it does seem more tedious than I expected.
Any ideas are greatly appreciated.
Monday, January 24, 2011
How can I avoid felting wool when I dye it?
However, I have managed to get felting simply by slowly draining off the hot water through the tap. Is this sufficient to cause felting, or is the water temperature also a factor? I am not aware of the urn actually boiling as it has a built-in thermostat.
Friday, January 21, 2011
I have a dress made of acetate, nylon and spandex fabric that I want to dye...what is my best option?
Country or region: United States
Message: I have a dress made of acetate, nylon and spandex fabric that I want to dye...what is my best option? Thank you for your help!
Thursday, January 20, 2011
Looking for a way to piece-dye cotton/polyester garments with nylon zippers




Information about how to dye multi-fiber blends


John Shore's
Blends Dyeing


Country or region: The Netherlands
Message: As a textile (searching) agent, interested to find a way for the piece dyeing of cotton and polyester garments with a nylon zipper on a cotton or polyester tape, I visited your site. Although I have not yet found the solution, I want to communicate I am impressed by your knowledge and the quantity and quality of the information on your site. Good luck with your health and kind regards.
Wednesday, January 19, 2011
The link for "The Kind Dyes" does not work


(Please help support this web site. Thank you.)
Tuesday, January 18, 2011
Is there any way to bleach a formal outfit, then re-dye to the right color?
Country or region: USA (California)
Message: Hello,
I bought a raw silk wedding outfit from India that was supposed to be a deep crimson...still red, but a deep, rich color. If you've ever seen an Indian outfit, you know there is a lot of detail that is added onto the fabric.
The outfit turned out to be more maroon than crimson. Is there any way to "undo" or bleach the fabric, then re-dye? Will this process ruin the embellishments or re-dye the pieces unevenly?
Sunday, January 16, 2011
How long can I keep dye mix concentrate that has been cooled but NOT yet mixed with the dye powders?
Country or region: Australia
Message: I can't find an answer on the website. I am using dye concentrate (urea, thickener and hot water - cooled and then mixed with procion mx dye powder) how long can I keep the concentrate that has been cooled but NOT yet mixed with the dye powders. I know it lasts about a week when mixed with dyes but can I keep it indefinitely if not yet mixed with powders? I don't want to be wasteful and throw it out if I can use it again. Thanks.
Saturday, January 15, 2011
Friday, January 14, 2011
Where, on a trip to London, can I buy dyes and dye-related books?
Country or region: Portugal
Message: Please. I am going to London next month. A year ago I went to a batik course. I tried to find the dyers here in Portugal but without success. My trip is to go and buy dyes, there in London. Dyers, some books. I already bought the cantings from EUA, from Dharma. I have done some wax works. Please could you telI me where to go, a shop in London where I can buy special the dyes. Thanks a lot
Thursday, January 13, 2011
Vat dyes lose their sharpness and become dull
Country: Nigeria
Message: Hi there,
Hope this won't be too much of a bother but I am at my wits end.
I am self taught and have been dyeing for a few years now and I have noticed a recurring problem with my finishing. What I dye is primed canvas fabric and the preferable type of dye easily and cheaply accessible here is the vat dye. Once dyed the finished fabric looks sharp and bright but after a couple of uses the fabric loses its sharpness and becomes dull. I have tried using scotch guard fabric protector on the fabric hoping it will help in fixing the dye but the fabric still looks dull after a while. What would you suggest I do? Your advice will be greatly appreciated seeing as you are an expert in the field. To get a clear picture of how I mix my "chemicals" I use 2 times more hydrosulphite than Soda eg. 2 tablspns soda and 4 tblspns Hydros and probably 4 or more full tspns of vat dye. I let my waxed fabric soak for at least 6 hrs and when rinsing, the dye stops running after 2 or 3 tries.
I am eagerly awaing your response and I will be happy to answer any questions you might have.
Tuesday, January 11, 2011
Are the bonds that connect dye and fabric polar covalent or non-polar covalent?


Linda Knutson's
Synthetic Dyes for Natural Fibers




Monday, January 10, 2011
Can you recommend a dye I can use for plastic six-pack soda rings?
Country or region: USA
Message: I like to dye plastic. Like the plastic six-pack soda rings. Can you recommend a dye I can use? I brought Tinfix, but it's for silk and it doesn't seem to take. I also brought iDye, but haven't tried it yet. Hopefully that would work. The Rit dye did not work either. I've seen it done before. Please help!
Saturday, January 08, 2011
Color sequences for 'dip dyeing' to get color changes for a series of colors



Message: I searched for color sequences for 'dip dyeing' to get color changes for a series of colors. I have one for yellow to red to burgundy, but need one for yellow/blue/green, and for yellow/orange/red/purple.
pale fuchsia/pale lilac/blue/purple
If, instead, you choose to use green instead of blue, the small amount of red, from the original pale pink, will lend a somewhat brownish olive tone to any green that you mix:
pale fuchsia/pale lilac/olive green/brown/black
Does any of this help? Specific dye color recommendations would depend on the class of dye you're using; both ProChem and Jacquard sell many different types of dye that work on silk, both several types of acid dyes and several types of reactive dyes reactive dyes.
Thursday, January 06, 2011
Is a Sharpie marker all right for coloring an embroidered logo?
Country or region: Anderson, S.C.
Message: I had a solid white hat with a Steelers emblem embroidered on it. I took sharpie markers and colored in the logo. I am planning to let the hat dry for a week. I'm not planning on washing the hat or getting it wet. It is not cotton. Just wondered if you thought I'd be all right. Thanks for your time & opinion.
Keep the marker, if you can, in case you want to do a touch-up later.
(Please help support this web site. Thank you.)
Tuesday, January 04, 2011
Where can I order 100+ small, medium, and large plain white cotton shirts?
Country or region: USA, Washington
Message: I love the site! I'm going to make up 100+ tye dye shirts and sell them at the fair. Where can I order 100+ small, medium, and large plain white cotton shirts?
Saturday, January 01, 2011
Is Procion MX dye going to be enough to penetrate sheets with a washing machine dye process?
Country or region: USA
Message: Hello and thank you so much for this page.
I have some egg shell colored white 1000 count sheets and we want to dye them.
Is Procion MX dye going to be enough to penetrate the sheets fabric with a washing machine dye process?
Thanks again for the web page!
How can I dye faded car mats back to their original color?
Name: Marcus
Country or region: Australia
Message: Hello, I have some original OEM car mats out of a car I am restoring. They have faded/worn to a bluish colour. I don't know what material they are made of, but they are a thick almost shag pile carpet backed with rubber. They are a valuable item and no longer available so I want to restore them to black. I have tried using iDye Poly (black), but this was unsuccessful. This was most likely due to the fact I was unable to get enough heat into the process. The mats are heavy and large, so I can't just put them in a pot on the stove. I used hot tap water and let sit over night, but the final result was poor. Would you be able to recommend a dye/process that might allow me to return these mats to black?
There are four possibilities that don't involve immersing the mats in a huge cooking pot for boiling. None are ideal, but perhaps one would be better than what you have right now. My favorite of these ideas is the last one, but I can't be certain that its results will look good.
You'll want to test any of these on a less valuable material before risking your original mats. You should also take pains to thoroughly clean the mats before applying any treatment to them, since even invisible stains can resist dyes or paints, resulting in uneven coloring.
Here are the four options to consider:
1. Disperse dyes, such as the dyes in your iDye Poly, must have high heat to transfer, much hotter than hot tap water, but, for some forms of the dye, the heat can be provided by a hot iron, instead of immersion in boiling water. The crayon effect of the disperse dye in Crayola Fabric Crayons is probably not what you're looking for, but they are very cheap and relatively easy to find in local stores, so they might be a good test. (See Iron-on Fabric Crayons for Synthetic Fibers.) If they work for you, you could then consider ordering disperse dyes for transfer from a company such as Batik Oetoro or Kraftkolour, both of which are located in Australia and do mail-order sales (or, in the US, PRO Chemical & Dye). It would take a lot of work to get a fairly even black color, involving many repetitions of the ironing on. What I'm most worried about is the question of whether the heat of a hot dry iron might damage your mats, either the pile of the carpeting or, in particular, the rubbery backing. If you test this, do it on the most inconspicuous part you can find on the back part of the mat. (Is there any possibility that you could find similar mats that are no longer any good, for testing your methods?)
2. A thin fabric paint, such as Jacquard's Dye-Na-Flow, could possibly be used to color the pile of the carpet, but it would not work on the rubbery backing. Fabric paint is better than other paints because it is softer and does not create the scratchy stiffness of regular paint that's not intended for use on fabric. The fact that it won't work on the rubbery backing is a big problem.
3. Krylon Fusion for Plastic is a spray paint that works much better on most plastics than other types of paint. It might work for the rubbery part of your mats. It will coat over the carpet pile, however. It might help to work it in with a stiff brush, such as a toothbrush, quickly, after spraying, before it dries, but it's not something I can recommend without careful testing. (If you can't find mats just like the ones you're trying to color, as a test, maybe a trial on a scrap of carpet would help; ask for a scrap at company that sells carpet installation.)
4. One other product might be worth testing. It's called vinyl dye, although it's not really a dye but instead a type of paint. The pigments in vinyl dye penetrate a short distance into the plastic it's painted on, which means that it doesn't chip off the way other types of paint do. Unlike true dyes, vinyl dye is somewhat opaque, so much so that lighter colors can be used after applying a base coat of white. I have not tried vinyl dye, and I have no idea what it will do on pile carpeting. It is recommended for use on many different surfaces, including hard vinyl, carpet, and even leather. You can find this product in an auto parts store. Given that the label claims it can be used on both plastic and on carpet, this seems to me like it might be your best bet.
Please do not try any of these ideas without first testing them on a bit of scrap carpet of some sort, as similar as possible to the material you want to color. And please do let me know how it works out, especially if you try the vinyl dye.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Friday, January 28, 2011
My question is how to dye silk cocoons without heat, as it softens them too much
Name: Linda
Message: Thank-you so much for your blog and site--I am always learning.
My question is how to dye silk cocoons without heat, as it softens them too much. I am doing a very large hanging of over 2000 and have already removed the worm. Each cocoon is in excellent condition and I want to keep them that way.
I have tested a few with dynaflow --I quickly dyed them, put them in a salad spinner and then under a heat lamp--this all worked great but it does seem more tedious than I expected.
Any ideas are greatly appreciated.
There are four possibilities for dyeing silk cocoons without heat, with different drawbacks. You will want to test to see which is the best method for you:
1. You can use a cool water fiber reactive dye, such as Procion MX, so that you don't have to heat the cocoons at all. This is the method recommended by Jacquard Products.
Procion MX dyes can be used on silk at a room temperature of 70°F or higher, if you use a high pH. The usual method to raise the pH of the dye is to add soda ash, either directly to the dye immediately before use, or by presoaking the material to be dyed in dissolved soda ash for five to fifteen minutes. The latter is the standard recommendation for tie-dyeing and dye painting; to get started, I recommend that you buy a good tie-dyeing kit, such as the ones sold at local crafts stores under the Jacquard Products name. (Avoid the Rit tie-dye kit, which contains only hot-water dyes, dyes which happen not to be at all suitable for most tie-dyeing.) You can put the dyes into squirt bottles and apply them directly to soda-ash-soaked cocoons in a wide range of different color combinations, if you like, or you can dye a large number to exactly the same color using a bucket dyeing recipe, in which the soda ash is added gradually only after the dye has had some time to soak into the cocoons, for solid even colors. Salt is needed for the bucket-dyeing recipe, but not for the direct dye application recipe.
2. If the first method doesn't work out for you, you can use the same Procion MX dyes, but with a mixture of baking soda and soda ash to produce a lower pH.
The high pH of soda ash in the first method may soften the cocoons. Please do a test to see whether this happens to a sufficient extent to be a problem. If it does, you can compromise by using a somewhat lower pH. The reaction between the dye and the silk will not be as efficient at a pH of 9 as it is at the usual pH of 10.5 or 11, so the colors may not be quite as intense, but it will still work; just allow a nice warm room temperature, use a high concentration of dye, and allow lots of time for the dye reaction to take place, perhaps leaving the cocoons damp with the dye for a couple of days. The way to get a pH of 9 is to use baking soda mixed with soda ash or washing soda. Baking soda alone will produce a pH of 8, which is less efficient still, though better than a neutral pH.
Since water supplies vary, you should buy some pH paper to test what pH you get with a given mixture of baking soda and soda ash or washing soda, but I found that a wide range of mixtures of baking soda and soda ash (sodium bicarbonate and sodium carbonate) produced a pH in this range. Our water here is very neutral in pH; when I mixed 5 grams of baking soda and 5 grams of soda ash in 250 milliliters of water (about half a teaspoon of each in one cup of water), I got a pH of 9. So, that's a good place to start, even without pH paper.
3. You can use acid dyes, which are available in a wide range of types. The mild vinegar used as an assistant with the acid dyes has a low pH, which is kinder to silk than a high pH is. Unfortunately, all acid dyes work best with heat. Heating helps in the formation of the hydrogen bonds that attach the acid dye to the fiber. There are some recipes that call for using acid dyes without heat, by extended soaking. I expect this method to be less successful than using Procion MX dyes at a pH of 9.
4. The fourth method is a variation on the one you've already tried, using a fabric paint. Dye-na-flow is a paint, not a dye, which is why it can be set by dry heat, instead of moist heat like acid dyes. Instead of the heat lamp, you can choose to add an acrylic catalyst to the paint, immediately before use, to make it set without heat, which would make the method a little less tedious. The catalyst product is called Jacquard Airfix. It's not easy to find, but one source is Jerry's Artarama, which does business by mail-order.
Please let me know how your tests work out, and which method turns out to be the best for your silk cocoons.
(Please help support this web site. Thank you.)
Please let me know how your tests work out, and which method turns out to be the best for your silk cocoons.
(Please help support this web site. Thank you.)
Monday, January 24, 2011
How can I avoid felting wool when I dye it?
I realize that dyeing wool fibre in a tea urn and agitating the fibre will cause some felting.
However, I have managed to get felting simply by slowly draining off the hot water through the tap. Is this sufficient to cause felting, or is the water temperature also a factor? I am not aware of the urn actually boiling as it has a built-in thermostat.
Felting is caused not only by agitation, but also by sudden changes in temperature. This is why, in dyeing wool, recipes advise only gradual changes in temperature, bringing up the temperature of the dyebath slowly from room temperature only after adding the wool to it, then, after the dyeing period is complete, allowing the dyebath to cool to room temperature before removing and rinsing the wool in room-temperature water.
It is okay to use quite high temperatures in dyeing wool, even bringing the dyebath to a boil, as long as the change in temperature is sufficiently gradual. Do not rinse hot wool in room temperature water, or put room temperature wool into a hot dyebath, because you want to avoid the shock of the sudden temperature change.
Felting is also encouraged by exposing the wool to a high pH. It is important to dye wool under acid conditions, which is to say, at a low pH. What kind of dye are you using, and what else are you adding to your dyebath? There are some dyes which are sold already mixed with a high-pH chemical, designed for use in dyeing plant fibers, such as cotton. Silk is sturdier and can be dyed under these circumstances, but wool is sensitive to damage. Don't use a high-pH dye mixture, such as Dylon Permanent dye or Tulip Permanent dye, on wool. There are many different acid dyes that are suitable for use on wool; see my pages "About Acid Dyes" and "How to Dye Wool".
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Friday, January 21, 2011
I have a dress made of acetate, nylon and spandex fabric that I want to dye...what is my best option?
Name: Robin
Country or region: United States
Message: I have a dress made of acetate, nylon and spandex fabric that I want to dye...what is my best option? Thank you for your help!
That's not a good dress to dye, I'm afraid. Acetate should be dyed by heating it with disperse dye, while spandex should never be heated. The right way to dye that fiber blend is to dye the acetate yarns, nylon yarns, and spandex yarns separately, before weaving or knitting them together into a single fabric.
Spandex is heat-sensitive. Check the care label on your dress. It probably tells you that you should wash it only in cool water. Exceeding a temperature around 105°F is apt to make your dress lose its shape. Even for dyeing spandex before it's put together into a garment with a shape, you should never exceed 140°F. See my page,"How to Dye Spandex".
Acetate should be dyed by heating it with a special dye for synthetics, called disperse dye; home dyers can do this using a very large cooking pot on the stove. The desired temperature range for dyeing acetate is 180°F to 200°F, obviously far too hot to preserve spandex without damaging it.
Nylon is best dyed with acid dyes, but it can also be dyed with the same disperse dyes that work on acetate and polyester. It should be heated to about 185°F; higher temperatures may damage nylon, but lower ones will not result in optimal dyeing.
If you would like a tie-dyed or watercolor effect, particularly with multiple colors, then you should consider using fabric paint instead of dye. Choose a brand of fabric paint which is not supposed to leave a harsh feel on the fabric, and which is recommended for synthetic fibers; some fabric paints that are recommended only for natural fibers will not stick well to synthetic fibers. Two good possibilities would be Dye-Na-Flow, made by Jacquard Products, and Dharma Pigment Dyes, available only from Dharma Trading Company. Two warnings: you cannot use fabric paints to lighten the color of your dress, only to darken it, and you cannot get a perfectly smooth solid color.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, January 20, 2011
Looking for a way to piece-dye cotton/polyester garments with nylon zippers
Name: Peter
—ADVERTISEMENTS—
Dye polyester and poly/cotton blends
Information about how to dye multi-fiber blends

John Shore's
Blends Dyeing
Country or region: The Netherlands
Message: As a textile (searching) agent, interested to find a way for the piece dyeing of cotton and polyester garments with a nylon zipper on a cotton or polyester tape, I visited your site. Although I have not yet found the solution, I want to communicate I am impressed by your knowledge and the quantity and quality of the information on your site. Good luck with your health and kind regards.
I'm glad the site has been helpful to you. The answer to dyeing anything with polyester is to use disperse dyes. No other class of dyes will work. Although nylon is usually dyed with acid dyes, it, too can take disperse dyes, which work only on synthetic fibers. See my pages, "How to dye nylon" and "Dyeing Polyester with Disperse Dyes".
Cotton will not take disperse dye, so you must use a different type of dye for that, either reactive dye or direct dye. See "About Direct Dyes" and "About Fiber Reactive Dyes". Although direct dye suffers from relatively poor washfastness, so it fades relatively quickly when a garment dyed with it is laundered, it can be treated with a cationic fixative afterwards that will improve the washfastness to an acceptable level. Note that the boiling temperatures required to dye polyester will shrink any cotton significantly, a problem which has to be allowed for in advance.
There are two ways to go about dyeing a garment that contains both cotton and polyester. You can use the disperse dye and the cotton dye in separate steps, washing out the excess dye from the first step before beginning on the second. To do this, you would boil the garments with disperse dye. For intense colors, you will also need to use an added carrier chemical, which smells horrible and demands truly excellent ventilation, or to be applied in a dyeing pot on top of a cooking unit that can be used outside, such as a propane burner or electric hot plate. After completing the boiling, you would wash out any unattached dye, and then you could move on to the next step, using either reactive dye or direct dye to color the cotton portion of the zipper tape and/or garment. When using two distinct steps for multi-fiber dyeing, I prefer the use of fiber reactive dyes, such as Procion MX, Drimarene K, or Remazol dyes, rather than the use of direct dye, because the performance of reactive dyes is very good.
Alternatively, another method is to use direct dye for the cotton, and apply it in the very same dyebath as the disperse dye for the polyester and nylon, at the same time. This is the method encouraged by Jacquard Products with their pair of dye lines, the "iDye" (direct dye for cotton and rayon) and the "iDye Poly" (for polyester, acrylic, and other synthetics). If you know the correct amounts to use, you can use any direct dye and disperse dye in the same fashion. Fiber reactive dyes are not suitable for application in the same dyebath with disperse dyes.
It's often a challenge for an artist or craftsman to find small quantities of the dyes required for this project. If you are working with larger quantities, in industry, you can order five-kilogram barrels of each dye color directly from a manufacturer such as Dystar, after first requesting small samples for your testing. If you are working with smaller quantities, you might be interested in the sources that dye artists and craftsmen use. Look at the "Europe" section of my page, Sources for Dyeing Supplies Around the World. The dye suppliers listed there will ship dye to other countries, and there is one well-regarded shop in the Netherlands, Zijdelings, which sells Procion MX fiber reactive dyes, and sells transfer dyes which contain disperse dye. You should describe your needs to them so that they can tell you whether or not their disperse dyes are suitable, and whether or not they also sell direct dyes. George Weil in the UK is among the suppliers who carry Jacquard Products' iDye and iDye Poly, and they also carry another line of disperse dyes, as well as needed additives, and both Procion MX dyes and Deka L direct dyes. (Jacquard's iDye Poly includes the carrier chemical additive in a separate packet inside the iDye Poly package.)
If you choose to use direct dye, including Jacquard Products' iDye, I strong recommend that you also use a cationic dye fixative aftertreatment, which makes direct dyes resistant to washing out. See my page, "Commercial Dye Fixatives" for more information. Your dye supplier should be able to provide this sort of treatment, in various brands. Depending on the regulations you must follow, you may need to specify one that is formaldehyde-free, unlike the brands available in the US, which all contain at least traces of formaldehyde.
A few warnings: note that the disperse dye might produce slightly different colors on nylon than on polyester. Proper testing is essential, before making any decisions as to what materials and methods to use. It is possible that yet a third type of dye, some suitable acid dye, will be required for your nylon; the most washfast dyes for nylon are in the acid dye class. Since nylon is sensitive to heat, it may be necessary to reduce the heat of the dyebath for dyeing the polyester to 85°C; any temperature reduction from boiling makes the dye carrier molecule even more important for reaching full color on the polyester. It may be a significant challenge to get a close enough color match between your disperse dye on polyester and your direct or reactive dye on cotton, which only trial and error can perfectly resolve. I cannot advise you on what techniques are used in industry, only on the small-scale methods used by hand dyers, which are based on the same principles.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Wednesday, January 19, 2011
The link for "The Kind Dyes" does not work
Name: Sue
Country or region: USA
Message: The link for "The Kind Dyes" does not work. I tried searching for it several different ways, so don't know if it has been taken down. It was on the page about tie dyeing. I appreciate the info you have since I'm a beginner.
It's sad to see that the site for The Kind Dyes has disappeared. They did very beautiful and unusual work. You can see some of it which has been saved for posterity via the Internet Archive. Look at the following links:
[front page of site]
It takes some time for all of the images to load. Some of the images are no longer available, but the ones that are there are well worth looking at. Check the list of archived results from different dates to see some different pictures.
I'm sure you'll want to see their page describing how they made their tie-dyes:

Thanks for writing. I am sorry that I will have to remove their link from the page you saw it on. I hope the artists from The Kind Dyes are still making their beautiful tie-dyes somewhere.

(Please help support this web site. Thank you.)
Tuesday, January 18, 2011
Is there any way to bleach a formal outfit, then re-dye to the right color?
Name: Anjali
Country or region: USA (California)
Message: Hello,
I bought a raw silk wedding outfit from India that was supposed to be a deep crimson...still red, but a deep, rich color. If you've ever seen an Indian outfit, you know there is a lot of detail that is added onto the fabric.
The outfit turned out to be more maroon than crimson. Is there any way to "undo" or bleach the fabric, then re-dye? Will this process ruin the embellishments or re-dye the pieces unevenly?
No, you really can't do this. Is the dress washable? Most formal outfits are not washable, and yet removing dye is a much harsher process than machine washing. The details of the outfit are very likely to suffer badly in the process. The trim might also be affected to a greater or lesser extent, so that it no longer matches the fabric. In addition, some dyes simply cannot be discharged, but there's no way to know in advance whether the manufacturers of the materials used in your outfit used dischargeable dyes. You might treat the fabric and then find that the color remains unchanged, no matter what you do to it.
There are chemicals that can be used to remove dye from silk. (See "What chemicals can be used to remove dye?".) You can't ever use chlorine bleach on silk, because the hypochlorite which is the active ingredient will destroy the silk fiber, but sulfur-containing dye discharge agents such as the sodium dithionite in Rit Color Remover, or the thiourea dioxide in Jacquard Color Remover, can be used on silk. You have to use hot water to make it work, though, and the best way to use it is by cooking the garment with the chemicals in a huge cooking pot on the stovetop until nearly simmering. It's hard to imagine a formal outfit that would not be destroyed by such treatment.
Even if it were possible to lighten the color of the outfit, dyeing would be a problem. It is very likely that some or all of the trim on the dress, and all of the stitching, is made of polyester or another synthetic fiber, which means that you cannot dye them to the same color at the same time. Although polyester can be dyed, it requires a different type of dye than silk does, and it requires such harsh conditions of boiling that the silk would be ruined. To dye raw silk for a formal outfit, you must dye the fabric separately before sewing.
Even if it were possible to lighten the color of the outfit, dyeing would be a problem. It is very likely that some or all of the trim on the dress, and all of the stitching, is made of polyester or another synthetic fiber, which means that you cannot dye them to the same color at the same time. Although polyester can be dyed, it requires a different type of dye than silk does, and it requires such harsh conditions of boiling that the silk would be ruined. To dye raw silk for a formal outfit, you must dye the fabric separately before sewing.
Your options are to 1, learn to love the outfit in its current color, or 2, get another one made in the color you want. The sad lesson is that one should never buy custom-made clothing sight-unseen, especially if the colors matter, unless it will be easy to return unacceptable items.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Sunday, January 16, 2011
How long can I keep dye mix concentrate that has been cooled but NOT yet mixed with the dye powders?
Name: Michelle
Country or region: Australia
Message: I can't find an answer on the website. I am using dye concentrate (urea, thickener and hot water - cooled and then mixed with procion mx dye powder) how long can I keep the concentrate that has been cooled but NOT yet mixed with the dye powders. I know it lasts about a week when mixed with dyes but can I keep it indefinitely if not yet mixed with powders? I don't want to be wasteful and throw it out if I can use it again. Thanks.
You can use it as long as it smells okay. When urea goes bad, it forms ammonia, which can interfere with the pH of your dyeing. Ammonia has a very strong and penetrating odor, so it's easy to detect.
PRO Chemical & Dye says that unused prepared print paste can be stored without dye up to six months, normally without refrigeration. Their print paste mix contains urea, the thickener sodium alginate and the water softener sodium hexametaphosphate; the latter is very important in preventing alginate from forming a thick and difficult-to-remove calcium gel if it is mixed with hard water, which contains calcium ions.
The Procion MX fiber reactive dyes themselves will last a lot longer if refrigerated after they are dissolved, as long as not even a small trace of soda ash or other high-pH chemical comes into contact with them. They will retain most of their strength for at least a month in the refrigerator, and possibly longer. Allow them to return to room temperature before using.
Also see:
- I'm wondering whether to store the mixed dye in the fridge or at room temp. (blog post from August 13, 2005)
- shelf life after refrigeration (Dye Forum discussion from August 2009)
Saturday, January 15, 2011
Name: deb
Country or region: USA
Message: How much would you charge for 5 dozen tie-dyed yamulkas?
I can't take on your yarmulke-dyeing project for you, but tie-dyeing yarmulkes is popular these days. Dharma Trading Company is now selling easy-to-dye cotton yarmulkes, which you could contract with a custom tie-dyer to dye for you. You could also buy a good tie-dyeing kit, such as the ones also sold by Dharma, and do it yourself, as many people do.
To find a custom tie-dyer to dye your yarmulkes, see my page entitled "Where can I find someone to dye my clothing for me?"; call or e-mail the custom tie-dyers listed to ask about prices and about how much lead time is required.
Alternatively, you could post a request on Etsy's Alchemy service.

Here is a link, "Tie-Dye Kippot/Yamulkas/Skullcaps," to pictures of work done by one of the members of the Dye Forum on this web site, Elisheva Ben Ze'ev. See her contact information at www.Groovesters.com if you would like to request a quote from her for your project.

Here is a link, "Tie-Dye Kippot/Yamulkas/Skullcaps," to pictures of work done by one of the members of the Dye Forum on this web site, Elisheva Ben Ze'ev. See her contact information at www.Groovesters.com if you would like to request a quote from her for your project.
(Please help support this web site. Thank you.)
Friday, January 14, 2011
Where, on a trip to London, can I buy dyes and dye-related books?
Name: Filomena
Country or region: Portugal
Message: Please. I am going to London next month. A year ago I went to a batik course. I tried to find the dyers here in Portugal but without success. My trip is to go and buy dyes, there in London. Dyers, some books. I already bought the cantings from EUA, from Dharma. I have done some wax works. Please could you telI me where to go, a shop in London where I can buy special the dyes. Thanks a lot
How exciting! I've never been to London, but I do have some information on dye suppliers in the UK, to get you started. Use a web mapping service such as Google Maps to look each one up, to find if any will be near the areas you'll be staying in.
In general, dye prices are significantly higher in the UK that in the United States, especially on Procion MX dyes, and Lanaset dyes are almost not available at all. Take a look at my page, "Sources for Dyeing Supplies Around the World". The second section, European Sources for Dyeing Supplies, contains the UK entries as well as other European dye suppliers. These are the UK suppliers in my list:
- George Weil / Fibrecrafts (UK - phone 0(+44) 1483 421853, fax 0(+44) 1483 419960; http://www.georgeweil.com, email esales@georgeweil.com). DEKA fabric paints and dyes, Procion MX type dyes, other dyes and fabric paints.
- Kemtex Educational Supplies (Chorley Business & Technology Centre, Euxton Lane, Chorley, Lancashire, UK PR7 6TE; http://www.kemtex.co.uk/; Tel: 0 1257 230220; Fax: 0 1257 230225) procion, disperse, direct, acid dyes, and many others. No credit cards or e-mail; fax and money-transfer.
- Omega Dyes (Cornwall, UK; http://www.omegadyes.co.uk/) Acid, direct, reactive, disperse ('transfer') dyes; 'Miracle Fix' for printing disperse dyes permanently onto natural fibers
- Rainbow Silks (UK - 6 Wheelers Yard, High Street, Great Missenden, Bucks HP16 0AL; http://www.rainbowsilks.co.uk/; Tel: +44 (0)1494 862111, Fax: +44 (0)1494 862651) Jacquard Procion dyes, many silk dyes and paints, illuminant dye to use with discharge paste, blueprint fabric, disperse dye.
- Textile Techniques (Shropshire, UK; http://www.textiletechniques.co.uk/). Javanese batik tools including many styles of cantings (tjantings), copper cap (tjap) stamps. Also batik classes, Procion MX type dye.
George Weil is not very far from London, 33 miles southwest of the center of London, at Old Portsmouth Road, Peasmarsh, Guildford, GU3 1LZ. However, Kemtex Educational Supplies is very far away in Lancashire, 200 miles to the northwest. Omega Dyes is far away, too, 110 miles to the west of London. Rainbow Silks is 39 miles northwest of the center of London at 85 High Street, Great Missenden, Bucks. Textile Techniques is almost 200 miles away, in Shropshire. It looks as though George Weil and Rainbow Silks are your best bets from that list.
Not included in the above list, C&H sells a full range of Dylon brand dyes, which are good dyes but less suitable for advanced dyers than other types of dyes, in Guildford, 30 miles southwest of the center of London, at 6a Tunsgate Square, Guildford, Surrey, GU1 3QZ.
Here are some additional dye and textile-related things to consider seeing in London and nearby cities....
- There is a Handweavers Studio and Gallery in London, located at 140 Seven Sisters Road, London N7 7NS, "a treasure trove for weavers, spinners, felters, dyers, embroiderers … in short, for textile artists, threadheads and fibre fiends of all persuasions", apparently also with a shop for dyes and other textile-related items. Check to see whether they sell books on dyeing, as well.
- The Fashion and Textile Museum in London "is a cutting edge centre for contemporary fashion, textiles and jewellery."
- The Lesley Craze Gallery in London "is an internationally recognised showcase for contemporary jewellery, metalwork and textiles."
- Foyle's bookstore in London is said to have a wonderful collection of books on textiles that are not widely available. An even more highly-regarded bookstore for textile books is R D Franks, close to the London College of Fashion and near Oxford Circus. See, for example, their webpage about dyeing & printing books.
- The Textile Society has textile-related events and exhibits, some in London, and some in February.
- Of course, many of the larger museums have interesting textile-related exhibits, such as at the Victoria & Albert Museum.
- The Pitt Rivers Museum, 60 miles to the northwest of London, in Oxford, has a Textiles collection.
- Silk painter Isabella Whitworth will be offering a class, "Dyes to die for", at Denman College, which is 70 miles west of London, at Marcham, Abingdon, Oxfordshire, 17th - 20th February 2011.
I hope you have a wonderful trip to London, and find exactly what you're looking for.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, January 13, 2011
Vat dyes lose their sharpness and become dull
Name: Amina
Country: Nigeria
Message: Hi there,
Hope this won't be too much of a bother but I am at my wits end.
I am self taught and have been dyeing for a few years now and I have noticed a recurring problem with my finishing. What I dye is primed canvas fabric and the preferable type of dye easily and cheaply accessible here is the vat dye. Once dyed the finished fabric looks sharp and bright but after a couple of uses the fabric loses its sharpness and becomes dull. I have tried using scotch guard fabric protector on the fabric hoping it will help in fixing the dye but the fabric still looks dull after a while. What would you suggest I do? Your advice will be greatly appreciated seeing as you are an expert in the field. To get a clear picture of how I mix my "chemicals" I use 2 times more hydrosulphite than Soda eg. 2 tablspns soda and 4 tblspns Hydros and probably 4 or more full tspns of vat dye. I let my waxed fabric soak for at least 6 hrs and when rinsing, the dye stops running after 2 or 3 tries.
I am eagerly awaing your response and I will be happy to answer any questions you might have.
It's sadly true that Scotchgard does not fix vat dyes. Neither do Retayne and other cationic dye fixatives, which work so well to improve the washfastness of cheap direct dyes. There is no dye fixative that you can add to fix vat dyes in place after dyeing, since their fixation must occur during the dyeing itself, not afterwards. I think that the answer in your case may lie in the step known as "soaping off", which is done after the last washing, but there are other possibilities worth examining, as well.
Vat dyes work differently from most other types of dye. In order to make them truly dissolve, you must chemically reduce them, which is the exact opposite of oxidizing them; you must exclude oxygen, as far as is practical (avoid vigorous stirring, which may introduce too much air to the dyebath), and instead use a chemical that will add electrons to the dye molecules. As a result, the dissolved dye particles can penetrate inside the fiber of the fabric or yarn. After this, when you expose the wet dyed fabric to air, the oxygen in the air acts to oxidize the dyes, turning them back to their insoluble state. Since, at this point, the dye particles cannot dissolve in water, those that are located inside the fiber are stuck fast and will remain there.
Unfortunately, some of the dye particles at this point are instead found on the surface of the individual textile fibers, not stuck inside at all; the step known as "soaping off" helps to remove this non-fixed vat dye after dyeing. If the non-fixed vat dyes are not removed, they give only a temporary effect of brightness, but they will tend to fade quickly, and, worse, to rub off onto other items even when dry, a serious dyeing fault known as "crocking". If too much vat dye is applied in one dip, more than is called for by the recipe, then, again, too much of the dye particles will remain on the outside of the fibers, instead of being fixed within the fibers; for more intense colors, it is better to do multiple dips with a weaker dyebath than to do a single dip in a dyebath that contains too much dye.
The first question to consider is always the fabric. Do you know that your fabric is 100% cotton (or other natural fiber), and free of surface finishes such as stain resistance? You have probably already made sure of this, but it's always worth asking. You can't get good bright colors with vat dye on a fabric that has a significant content of synthetic fiber, and I have seen canvas made with synthetic fibers such as acrylic. What do you mean by "primed canvas fabric"? Priming can be a problem, if it means anything other than thorough cleaning. Canvas that is primed for use by oil painters has been coated with a sort of glue that will prevent good dyeing.
A related question is your preparation of the fabric. Step one, as always with any dye, is to prepare the fabric by washing it in the hottest available water, preferably 140°F or higher (60°C), using laundry detergent plus some extra soda ash for added cleaning power. It is most important to remove any finishing chemicals that are on the fabric, as they dull the colors of any dye you apply, by preventing the dyes from fully penetrating the fiber. Some such finishes cannot be removed by washing, but the spinning oils and many sizings can be. If your fabric has been sized with starch, it will be impossible to remove it all, and the starch can interfere badly with dyeing.
Most of the vat dye recipes I see call for a certain number of grams of each ingredient, by weight, rather than the volume measures to which you are accustomed. You will get much more repeatable results if you can weigh your materials instead of using spoonfuls, since one lot of dye or any other chemical may be more or less dense than another. A certain number of grams is always the same amount, but the number of tablespoons per gram may vary. Would it be possible for you to buy a scale that is good for measuring quantities as small as one gram? I recommend that you do so, if you can find one.
When you mentioned soda, did you mean soda ash, or caustic soda? Soda ash contains sodium carbonate, or Na2CO3; washing soda is another form of sodium carbonate and can be used for the same purposes as soda ash. Caustic soda is a completely different chemical, also know as sodium hydroxide, or lye, known by the chemical abbreviation NaOH.
All of the recipes I know for using synthetic vat dyes call for the use of caustic soda. Soda ash (or washing soda) does create a high pH when you add it to water, but caustic soda produces a significantly higher pH. If you are using soda ash, you should get better results with caustic soda. Caustic soda is a hazardous material and has caused deaths among people who drank it, and blindness to those who splashed it in their eyes, so care is needed. It's safe to use with appropriate precautions. Expect the lye to make the water very warm when it dissolves. Wear waterproof gloves and safety glasses, and always add lye to water, never water to lye, and of course always keep it out of reach of children. Carefully label all bottles and jars that contain lye, and never store it near food or drink. Do not use reactive metal containers, such as aluminum, when working with lye, or soda ash or washing soda, because these high-pH chemicals will react with most metals. Stainless steel and enameled steel are both good.
A good way to troubleshoot your difficulties with vat dyeing is to look at some good recipes and compare your techniques. If your dye manufacturer has not supplied you with reliable instructions, look to other companies to see what they have. Start with my page, "About Vat Dyes". I like the detail of PRO Chemical & Dye's "Immersion Dyeing using PRO Vat Dyes" recipe [PDF] , but it calls for thiourea dioxide (Thiox), instead of the sodium hydrosulfite (Hydros) that you are using. It is necessary to use five times as much sodium hydrosulfite as thiourea dioxide, if they're reasonably pure; if either one is sold in a preparation that includes other chemicals such as sodium carbonate, measurement will be more complicated.
Aljo Mfg, in New York, has a simpler vat dye recipe, which calls for both caustic soda and sodium hydrosulfite, and for measuring with a tablespoon, as you have been doing, rather than by weight. For one pound (500 grams) of cotton or rayon fabric, they say to dissolve one tablespoon (15 ml) of caustic soda in a cup (240 ml) of cold water, then, in this mixture, dissolve a tablespoon of sodium hydrosulfite. In a separate container, mix one to two tablespoons of vat dye with a little warm water. Add the dye to 12 liters of warm water (30°C to 50°C). This is your dyebath. They say to add your pre-wetted fabric to the dyebath and add the lye/hydrosulfite solution, then raise the temperature of the dyebath to between 50°C and 70°C, stirring but not allowing the heat to approach boiling. Clearly you don't want to heat your dyebath to a high enough temperature to melt your wax, but they say "in batik, where lower temperature is used, dyeing time will be longer", so evidently this recipe can be used for your circumstances.
After dyeing with vat dyes, industrial recipes call for a "soaping off" step. This comes after you have completed dyeing and have washed out the fabric and exposed it to air to oxidize the dye to its final color. Apparently, in spite of the name, soap is not as important to this final step as the nearly boiling temperature of the water, suggested in some sources as being 95° to 98°C. Here is an explanation from J. Richard Aspland's 1997 book, Textile Dyeing and Coloration, p. 59:
"After sodium leuco-vat anions have diffused into cellulosic fibers, after the excess alkali and reducing agents have been washed off, after the oxidation has been accomplished and the pigmentary form of the vat dye is left embedded within the cellulosic fibers, then comes soaping.
"Soaping is peculiar to vat dyes and it is a misnomer in that it does not require the use of soap. It can describe any treatment, in near boiling aqueous solutions containing a surfactant, during which the isolated molecules of vat pigments are reoriented and associate into a different, more crystalline form. This new form can have a significantly different shade and gives the dyed goods materially improved fastness to light and washing."
Since you are applying wax, you may already be using a boiling water treatment at the end to remove the last traces of wax. If not, it's something to consider trying, since it is supposed to improve lightfastness and washfastness.
Your question is an interesting one. I hope that you will find this useful.
Also see the related question, "Safety of the caustic soda and hydro-sulphite used for tie-dyeing in Nigeria", posted in this blog on December 03, 2009.
Tuesday, January 11, 2011
Are the bonds that connect dye and fabric polar covalent or non-polar covalent?
Question: Are the bonds that connect dye and fabric polar covalent or non-polar covalent?
—ADVERTISEMENT—

Linda Knutson's
Synthetic Dyes for Natural Fibers
Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
The type of bond between dye and fiber depends on the type of dye. Different classes of dye attach to fibers via quite different forms of bonding. Most types of dye do not form covalent bonds to the textile fiber.
Fiber reactive dyes are the only dyes that form covalent bonds with the fiber, effectively becoming one molecule with it. Before the dye reaction, the reactivity of the fiber reactive dye molecule relies on polar covalent bonds.
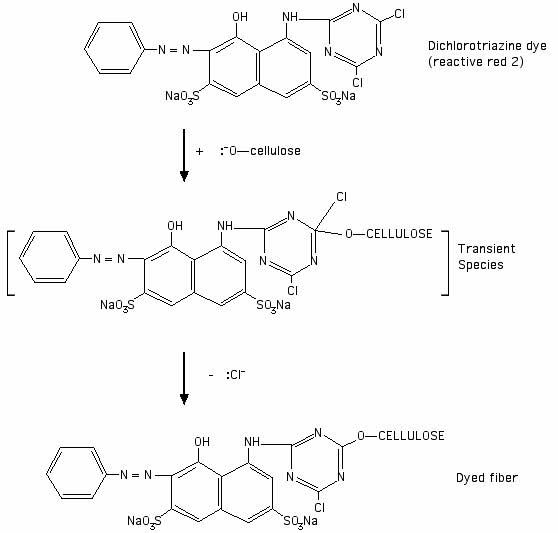
Using a dichlorotriazine dye (Procion MX dye) as an example: before the dye reacts with the fiber, the dye structure contains two chlorine atoms. The electronegativity of the chlorine atoms makes the dye susceptible to nucleophilic attack on the adjacent carbon atoms. Cellulose is activated to the nucleophilic cellulosate anion form by a high pH, as in the presence of soda ash, and then attacks one of the carbons that is attached to a halogen ion. See the drawing below as an illustration of the reaction:
[Drawing based on an illustration of a generic dichlorotriazine reaction in Chapter 4 of John Shore's 1995 book Cellulosics Dyeing, published by the Society of Dyers and Colorists.]
After the reaction has occurred, the halogen atom is replaced by the cellulosate. The carbon on the ring structure is attached to a nitrogen on either side in the ring within the dye itself, and to the oxygen of the cellulose molecule. From your studies so far, you should now have enough information to decide whether the covalent bond between the cellulose and the carbon atom to which it is attached is polar or non-polar, based on the respective electronegativities of carbon and oxygen atoms.
Most dyes do not form any sort of covalent bond with the fiber, whether polar or non-polar. Look at my page, "What kinds of chemical bonds attach dyes to fibers?". The only covalent bonding between dye and fiber occurs in the case of the fiber reactive dyes. These dyes are typically used for cellulose fibers, though they can also be used on protein fibers.
Acid dyes, which are normally the preferred choice of dyes for protein fibers, as well as for nylon, are attached to the fiber through hydrogen bonding and salt linkages. Direct dyes, which are the most inexpensive dyes available for plant-based fibers, loosely associate with the fiber through the dye property called substantivity, which relies on Van der Waals forces and some hydrogen bonding. Disperse dyes, which are used for synthetic fibers such as polyester and acetate, are dispersed through the fiber by vaporization from high heat, and are not bonded to it at all. Vat dyes, most popularly including the synthetic indigo used to dye blue denim, are converted to a soluble form and allowed to lodge inside the fibers before being returned to an insoluble form; naphthol dyes are held in the fiber by insolubility in much the same way. Mordant dyes, including most natural dyes, do not attach directly to the fiber at all, but instead form a complex to a metal ion which forms a bridge between the dye and the fiber. For more details, see my page, "What kinds of chemical bonds attach dyes to fibers?".
Please be careful to properly cite your sources in your schoolwork, as in the following citation for this article:
Burch, Paula. "Are the Bonds That Connect Dye and Fabric Polar Covalent or Non-polar Covalent?"Paula Burch's All About Hand Dyeing. 11 January 2011. <http://www.pburch.net/dyeing/dyeblog/C1845207367/E20110111074828/index.html>(Please help support this web site. Thank you.)
Monday, January 10, 2011
Can you recommend a dye I can use for plastic six-pack soda rings?
Name: Jennifer
Country or region: USA
Message: I like to dye plastic. Like the plastic six-pack soda rings. Can you recommend a dye I can use? I brought Tinfix, but it's for silk and it doesn't seem to take. I also brought iDye, but haven't tried it yet. Hopefully that would work. The Rit dye did not work either. I've seen it done before. Please help!
In dyeing plastic, it's important to know what KIND of plastic you have. Different types of plastics can be dyed with different dyes, except for a few plastics that can't be dyed at all.
For example, you can dye nylon plastics and polyurethane by heating them in a dyebath with acid dye along with an acid such as vinegar. (See How to dye nylon.) This is why Rit All-Purpose dye works on some plastics, because Rit contains an acid dye (mixed together with a different type of dye that does not work on any form of plastic). Similarly, Tinfix will, in many cases, work on nylon plastics, because many (though not all) of the dyes used in the Tinfix line of silk dyes for painting are acid dyes. However, acid dyes do not work on most other types of plastic, which, as you've seen, just remain white.
Plastic six-pack drink yokes are made from low-density polyethylene, LDPE, which is marked as a 4 inside a recycle logo.  LDPE is much more difficult to dye than nylon is, as it is chemically closely related to polyester. Polyester cannot be dyed with any dye that works on natural fibers. Your only option is to use disperse dye. Disperse dye is used to dye synthetic fiber materials such as polyester, acetate, and acrylic. (Interestingly, disperse dye also works on nylon, though the acid dye that works best on nylon will work on none of these other synthetic fibers.)
LDPE is much more difficult to dye than nylon is, as it is chemically closely related to polyester. Polyester cannot be dyed with any dye that works on natural fibers. Your only option is to use disperse dye. Disperse dye is used to dye synthetic fiber materials such as polyester, acetate, and acrylic. (Interestingly, disperse dye also works on nylon, though the acid dye that works best on nylon will work on none of these other synthetic fibers.)
 LDPE is much more difficult to dye than nylon is, as it is chemically closely related to polyester. Polyester cannot be dyed with any dye that works on natural fibers. Your only option is to use disperse dye. Disperse dye is used to dye synthetic fiber materials such as polyester, acetate, and acrylic. (Interestingly, disperse dye also works on nylon, though the acid dye that works best on nylon will work on none of these other synthetic fibers.)
LDPE is much more difficult to dye than nylon is, as it is chemically closely related to polyester. Polyester cannot be dyed with any dye that works on natural fibers. Your only option is to use disperse dye. Disperse dye is used to dye synthetic fiber materials such as polyester, acetate, and acrylic. (Interestingly, disperse dye also works on nylon, though the acid dye that works best on nylon will work on none of these other synthetic fibers.)Some crafts stores carry disperse dye in the form of Jacquard Products' dye, "iDye Poly". Don't confuse it with their plain "iDye", which works only on natural fibers; "iDye" and "iDye Poly" are often sold on the same rack, for the convenience of those combining the two types of dye to color cotton/poly blends. Do not try to use plain "iDye" on plastics; if that's what you have, go back to the same store and look closely for "iDye Poly", because plain "iDye" can be used only on natural fibers.
You can also find disperse dye in the form of fabric crayons, which work only on synthetic fibers; crafts stores and sewing stores often carry these under the name of "Crayola Fabric Crayons" or "Dritz Fabric Crayons". For a wider range of colors, and for more versatile formulations that can be applied in different ways, you can mail-order disperse dyes from PRO Chemical & Dye in Massachusetts, or from Aljo Mfg in New York. (See "Sources for Dyeing Supplies Around the World".)
Your problem is going to be heat. Disperse dye cannot be applied at room temperature, or even at merely warm temperatures. Either you will have to use the heat of a hot iron to transfer the dyes to your plastic from paper, or you will have to immerse the plastic in a pot of boiling hot water with the dye mixed in. (You will want to use a very large cooking pot, preferably either a stainless steel stockpot or an enameled steel canning pot, which you do not plan to reuse for cooking, since most textile dyes are not considered to be safe in food.)
The problem with applying heat is that your plastic yokes may suffer from the heat required to get the dye into them. You're going to have to experiment. Industry sources say that the maximum temperature LDPE can withstand is 80°C, or 176°F. Above that temperature, you may see the plastic begin to shrink or deform. Whether this is a problem or not will depend on what uses you'll be making of your dyed plastic, as well as how long it takes for the dye to penetrate into the plastic. According to an unreferenced claim in Wikipedia, LDPE can withstand temperatures as high as 95°C for a short time; polyester is normally boiled with disperse dye for at least half an hour, but some other materials can take up the dye more quickly or at lower temperatures. Acrylic fiber, for example, does not have to be boiled fully, as a temperature between 60°C and 70°C is sufficient for it to take up the disperse dye. It's a good idea to have a glass thermometer, which is more resistant to acids than a metal thermometer, so that you can keep careful notes on what dyebath temperatures work best for you. (If you don't have one already, PRO Chemical & Dye sells an appropriate glass thermometer, as do chemical supply houses.)
Dyeing polyester with disperse dye works best with an added chemical carrier, which is included in a separate packet inside the iDye Poly packet, or as a separate chemical known as Dye Carrier NSC from ProChem. It is difficult to get full color intensity on polyester at boiling temperatures without the dye carrier, as polyester dyes better at even higher temperatures. Other fibers, such as acrylic, do not require the dye carrier chemical at all. If you don't need to use the dye carrier additive, you'll want to avoid it, because it smells horrible and requires excellent ventilation. Do your tests on dyeing plastic yokes in a simmering hot disperse dye bath without the carrier first. If your color is not adequately intense, then try adding the carrier chemical to the dyebath and simmering longer. If possible, use the dye carrier chemical (plus the dyes) in a pot boiling on a portable burner outside, rather than inside your house. When I used it in my own kitchen, I ended up opening every door and every window, in addition to using a fan in the window, because the odor was so noxious. Disperse dyes themselves are not unpleasant or particularly toxic, but the carrier chemical is unpleasantly aromatic.
When you experiment with using a hot iron on your plastic yokes, to transfer color from paper that you've applied with disperse dye crayons or your own disperse dye paint, use enough protective material, such as a couple of layers of cotton rags or a piece of aluminum foil, to protect your iron from melting plastic, in case that happens. If you try the iron-on disperse dye crayons, or dye paint that you mix yourself from disperse dye powders, be sure to also protect your ironing board cover, as the dye can easily penetrate through a thin layer of paper or fabric.
For more information about using disperse dyes, see the following two pages:
"Dyeing Polyester with Disperse Dyes" and "Iron-on Fabric Crayons for Synthetic Fibers".
A very different alternative for coloring plastics, especially those plastics which cannot be dyed in any other way, is to use a special spray paint, Krylon Fusion for Plastics. It works better on hard plastics than other types of paint, penetrating a little farther into the surface of the plastic. The effect is not as good as a good dyeing job with a true dye, because it shows wear more quickly, but it is the best alternative if it turns out that dye simply does not work on a particular plastic material. Krylon Fusion for plastic is opaque, unlike dye, so that it can be used to change a darker color into a lighter one.
It works better on hard plastics than other types of paint, penetrating a little farther into the surface of the plastic. The effect is not as good as a good dyeing job with a true dye, because it shows wear more quickly, but it is the best alternative if it turns out that dye simply does not work on a particular plastic material. Krylon Fusion for plastic is opaque, unlike dye, so that it can be used to change a darker color into a lighter one.
(Please help support this web site. Thank you.).
A very different alternative for coloring plastics, especially those plastics which cannot be dyed in any other way, is to use a special spray paint, Krylon Fusion for Plastics.
(Please help support this web site. Thank you.).
Saturday, January 08, 2011
Color sequences for 'dip dyeing' to get color changes for a series of colors
Name: Linda
—ADVERTISEMENT—
Washfast Acid dyes
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on silk, wool, and nylon. One ounce of dye will dye six pounds of fiber!
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on silk, wool, and nylon. One ounce of dye will dye six pounds of fiber!
Country or region: USA, NY
Message: I searched for color sequences for 'dip dyeing' to get color changes for a series of colors. I have one for yellow to red to burgundy, but need one for yellow/blue/green, and for yellow/orange/red/purple.
I first mix yellow in pot, dip silk, remove it and add a bit of golden ochre and dip but not the entire piece, remove and add aztec gold, dip again, but keeping more of the silk out, then fire red, then burgundy and the effect is a great color transition. But I need to develop more color sequences, and are there any using black as the final color? I use ProChem, but do have some Jacquard dyes too.
That's an interesting color mixing question. It would be easy enough to have such a color gradation end in black. Having it end in a lighter color could be more problematic, depending on the color.
I am assuming, for all of this, that the entire piece of fabric gets colored the first color. You will have more color possibilities if you dip only part of the fabric into the first color (assuming a dye that is then fixed in place and that does not transfer to other parts of the fabric in a later dyebath, which depends on the type of dye you use), but that then would be a different question altogether (see how to dye a gradient or ombré).
The limitation is that you cannot go from one primary color to another, if you start by dyeing the entire piece your original color. The only way to go from one primary color to another is by leaving part of the fabric white in the first dyebath. However, you can always cover a light color with a much darker color.
As a general rule, you can move from a primary color to an adjacent secondary color. In dyeing, the primary colors are cyan, magenta, and yellow, just as in printing. Using a true red, royal blue, and yellow as mixing primaries can be done, but the results are never as bright, because a true red is already a mixture of magenta and yellow, and a true royal blue is already a mixture of a cyan plus a little bit of red or purple. So, if you mix a purple with a true red plus a royal blue, you will get a somewhat brownish purple, thanks to the combination of the yellow already included in the red color. This is a very good way to get more "natural" looking colors, for people who feel that the pure tones are too bright and artificial-looking.
You cannot go from yellow to blue to green. Blue plus yellow inevitably makes green. Instead, since yellow contains no blue, and blue contains no yellow, you must start with either yellow or blue and end in green. You can do this:
That's an interesting color mixing question. It would be easy enough to have such a color gradation end in black. Having it end in a lighter color could be more problematic, depending on the color.
I am assuming, for all of this, that the entire piece of fabric gets colored the first color. You will have more color possibilities if you dip only part of the fabric into the first color (assuming a dye that is then fixed in place and that does not transfer to other parts of the fabric in a later dyebath, which depends on the type of dye you use), but that then would be a different question altogether (see how to dye a gradient or ombré).
The limitation is that you cannot go from one primary color to another, if you start by dyeing the entire piece your original color. The only way to go from one primary color to another is by leaving part of the fabric white in the first dyebath. However, you can always cover a light color with a much darker color.
As a general rule, you can move from a primary color to an adjacent secondary color. In dyeing, the primary colors are cyan, magenta, and yellow, just as in printing. Using a true red, royal blue, and yellow as mixing primaries can be done, but the results are never as bright, because a true red is already a mixture of magenta and yellow, and a true royal blue is already a mixture of a cyan plus a little bit of red or purple. So, if you mix a purple with a true red plus a royal blue, you will get a somewhat brownish purple, thanks to the combination of the yellow already included in the red color. This is a very good way to get more "natural" looking colors, for people who feel that the pure tones are too bright and artificial-looking.
You cannot go from yellow to blue to green. Blue plus yellow inevitably makes green. Instead, since yellow contains no blue, and blue contains no yellow, you must start with either yellow or blue and end in green. You can do this:
yellow/chartreuse (slightly greenish yellow)/true green
Since navy blue is a very dark color, it can cover up any very light color, so you might be able to do this:
yellow/chartreuse (slightly greenish yellow)/true green/dark green/navy blue/black
though the navy blue is apt to be somewhat greenish.
You cannot have a base color of yellow and end up with purple, because yellow is the opposite color to purple. Purple on top of yellow makes brown. You could do this:
You cannot have a base color of yellow and end up with purple, because yellow is the opposite color to purple. Purple on top of yellow makes brown. You could do this:
yellow/yellow-orange/orange/red-orange/red
but from there your choices are limited to dark red, brown, or black—possibly all three, in that order.
If you start with blue, you can go in either the direction of green or the direction of purple. For green, overdye blue with either a yellow dye, or a green dye:
If you start with blue, you can go in either the direction of green or the direction of purple. For green, overdye blue with either a yellow dye, or a green dye:
blue/blue-green/green
from there you could add navy and then, optionally, black:
blue/blue-green/green/greenish navy blue/black
Starting with blue and adding magenta (or red), you can do this:
Starting with blue and adding magenta (or red), you can do this:
blue/blue-violet/purple
after which you have the option of adding a dark navy blue, for a navy-purple color, and then, if you wish, black.
You could start with a light pink made by diluting fuchsia, then add a light cyan or blue to make a pale lilac, then add a medium blue to make a blue that only shows the lilac a little, then to a dark blue or dark purple:
You could start with a light pink made by diluting fuchsia, then add a light cyan or blue to make a pale lilac, then add a medium blue to make a blue that only shows the lilac a little, then to a dark blue or dark purple:
pale fuchsia/pale lilac/blue/purple
If, instead, you choose to use green instead of blue, the small amount of red, from the original pale pink, will lend a somewhat brownish olive tone to any green that you mix:
pale fuchsia/pale lilac/olive green/brown/black
Does any of this help? Specific dye color recommendations would depend on the class of dye you're using; both ProChem and Jacquard sell many different types of dye that work on silk, both several types of acid dyes and several types of reactive dyes reactive dyes.
Thank you, what helped most is that yes it is ombre dyeing! I never knew it had a name. So I will try some of the color suggestions you listed below and see how it works.
Thanks again, color theory, for a not art trained person. What was I thinking, of course I cannot go from yellow to blue! or yellow to purple. Thanks.
Here are some links to discussions on the Dye Forum, on my site, about ombré or gradient dyeing:
Good luck with your dyeing, I'm sure you will be producing some beautiful silk.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, January 06, 2011
Is a Sharpie marker all right for coloring an embroidered logo?
Name: Scott
Country or region: Anderson, S.C.
Message: I had a solid white hat with a Steelers emblem embroidered on it. I took sharpie markers and colored in the logo. I am planning to let the hat dry for a week. I'm not planning on washing the hat or getting it wet. It is not cotton. Just wondered if you thought I'd be all right. Thanks for your time & opinion.
The Sharpie permanent marker was not a bad choice for your project. It's not dye, but the pigments stick well to the material, especially when not allowed to get wet (though they will resist a few washings pretty well). For items that will be washed, I much prefer markers that are labeled specifically as being permanent on fabric, but a Sharpie marker is good enough for an item that will almost never be washed.
The one warning that I would give you, if you want the color to last as long as possible, is to store the hat in the dark, or at least out of direct sunlight, when it's not in use or on display. Many markers and other pigments and dyes are susceptible to damage from visible light. This warning applies to a great many different sources of color, even high-quality dyes.
(Please help support this web site. Thank you.)
Tuesday, January 04, 2011
Where can I order 100+ small, medium, and large plain white cotton shirts?
Name: Marissa
Country or region: USA, Washington
Message: I love the site! I'm going to make up 100+ tye dye shirts and sell them at the fair. Where can I order 100+ small, medium, and large plain white cotton shirts?
If you want to order less than one case (72 shirts) of each size, then your best bet is the mail-order retailer Dharma Trading Company. They sell a huge variety of dyeable blank clothing, including a wide range of 100% cotton t-shirts. They provide quantity discounts for 12 and up, better discounts for 36 and up, and even better discounts for 72 and up. Better still, they will give you these discounts for assorted sizes, instead of requiring you to buy an entire 72-shirt case of each size in order to get any quantity discount.
You'll need to decide whether to buy organic or regular cotton; of course, organically-grown cotton is far more expensive than conventionally-grown cotton.
You must also decide whether you want to buy only shirts that have been sewn with cotton thread. Unless the retailer claims that a shirt has been sewn with cotton thread, all of the thread used to sew the shirts together turns out to be made of polyester, which will not dye at all with any dye that works to dye cotton. If you dye standard ordinary shirts sewn with polyester thread, then you will see bright white thread against the dyed fabric of your shirts. Cotton shirts sewn with cotton thread give a much more professional look. Look for "sewn with cotton thread" or "PFD" ("Prepared For Dyeing") or PFP ("Prepared For Printing").
Be careful to avoid shirts that contain any polyester, since it will not take the tie-dyeing dyes. Surprisingly often, the shirts sold by crafts stores next to the tie-dye kits are 50% cotton/50% polyester, which will not produce the brilliant results that a 100% cotton shirt can.
Some of the most popular t-shirt brands for tie-dyeing are Hanes, Gilden, Jerzees, Anvil, and Fruit of the Loom. My personal favorite is the Gilden Ultra Cotton shirt, just because it is sewn with cotton thread. Try ordering one shirt of each type from Dharma as a sample, to see which you prefer.
There are sources where you can find t-shirts of similar quality for lower prices than Dharma Trading Company, but the quantity discount does not kick in, in most cases, unless you order by the case, 72 shirts per size. Do a web search for "wholesale blank t-shirt". Check to see what the minimum order requirement is. You may need to get a wholesaler number from your state in order to get any discount, depending on the dealer.
Look carefully to make sure whether the shirts you are considering buying are 100% cotton and whether they are sewn with cotton thread. Also check to be sure that they are not lighter weight (in ounces of fabric per yard) than more expensive shirts, because flimsier thinner t-shirts should cost less and are not necessarily a good deal, depending on their price.
Do not buy any shirt that is promoted as being stain-resistant, wrinkle-resistant, or permanent-press, because the surface finish applied to the fabric to give these properties prevents the dye from reaching the fiber well enough. Stain-resistance and water-resistance are particularly bad for dyeing, since any treatment that resists water or stains will also resist dye quite effectively.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Saturday, January 01, 2011
Is Procion MX dye going to be enough to penetrate sheets with a washing machine dye process?
Name: John
Country or region: USA
Message: Hello and thank you so much for this page.
I have some egg shell colored white 1000 count sheets and we want to dye them.
Is Procion MX dye going to be enough to penetrate the sheets fabric with a washing machine dye process?
Thanks again for the web page!
Sheets are very dyeable, with a few caveats. The high thread count will not be a problem, and often the only way to get the color you want is by dyeing the sheets yourself.
First, there are some finishes, such as the formaldehyde used on 100% cotton sheets to make them less wrinkleable. (Doesn't it seem strange, to find that cotton sheets, which are heavily promoted as being "natural", are almost invariably coated with formaldehyde-containing resins?) These surface finishes can interfere with the access of the dye to the cellulose fiber. You will want to wash your sheets first in VERY hot water to remove as much as possible of any surface finishes. Not all will be removed, but my experience is that the overall results are usually just fine, anyway. Note that if you have not yet purchased your sheets, you should avoid any with claims of wrinkle resistance, no-iron, and the like, as sheets with those claims will have even more of the resin coatings than sheets that lack the claims on their packaging. They also tend to be less soft.
Check your washer to see if the "hot" setting allows cold water to be added in as the water fills; if so, turn off the cold water faucet, temporarily, as you fill the washer. Make sure your water heater is set to produce water that is 140°F, rather than being dialed down to 120° or even lower to prevent scald burns at the sinks. (Put warning notices next to the sinks while the water heater is turned up, if you think it's necessary.) Use Synthrapol or any other clothes washing detergent, usually no more than half the amount recommended on the label for most laundry detergents (people generally use too much), and add some extra soda ash (a.k.a. washing soda or sodium carbonate), as well, for extra cleaning powder without extra bubbles.
When you pre-wash your sheets, watch to see if they become twisted up by the agitator. If they do, you may need to untwist them repeatedly during the dyeing process. Twisted-up sheets cannot dye evenly; the regions inside the twists will receive less dye and end up a lighter color. Now, obviously a tiny pair of disposable latex gloves is not going to be completely adequate for reaching into a machine full of dye; prepare by buying yourself some ordinary sturdy rubber dishwashing gloves, or even the long-armed rubber gloves sold by Dharma Trading Company and PRO Chemical and Dye.
If your sheets contain any polyester content, for example, if they are 50% cotton and 50% polyester, only those fibers that are natural will take the dye. You can't dye polyester without boiling it (using a special polyester dye), which would be terribly impractical for an item as large as a sheet. Even the 50% poly sheets can dye very well if one allows for the paler color that will result from dyeing only half the fibers. Surprisingly, however, even 100% cotton sheets have stitching at the seems which is sewn with polyester thread. The polyester thread will remain the original color after you dye the sheets. This is not usually much of a problem, however.
Procion MX fiber reactive dye is your best choice for dyeing your sheets, whether they are made of cotton, linen, rayon, bamboo rayon, or modal rayon. It performs many times better than all-purpose dye. You will want to weigh your sheets while they are still dry in order to calculate how much dye you will need. For a dark color, you will need far more dye powder than for a light color. See my page, "How much Procion MX dye should I use?", for more information and advice on that question. You can mix different colors to get just the right shade, if none of the hundred different colors of Procion MX dyes available at the better dye mail-order retailers is quite right for your tastes. Note that the eggshell color of your sheets will cause any color of dye to come out a little bit yellower than you would get if you had pure white sheets. The results will be fine, but a blue might be a touch greener, or a red a touch oranger, so take that into account when choosing your colors.
You will also need perhaps twenty cups of non-iodized salt (table salt or pickling salt from the grocery store are ideal; if you use kosher salt, you will need to measure out more cups to get the same weight of salt, and how much you need will depend on what brand of kosher salt you buy, since one brand has crystals that occupy far more space, per gram of salt, then another does). See my page about salt for more details.
The third requirement is for sodium carbonate, also known as soda ash or washing soda, which provides the high pH required for the cotton (or other natural fiber) to react with the dye. You can order this with your dyes, or buy it at a hardware store or swimming pool supply store. See my page entitled, "What is soda ash, and what's it for in dyeing?".
Some dye recipes also call for a small amount of Calsolene oil, a wetting agent that helps dye to penetrate. I've done well in dyeing sheets without Calsolene, but it's a good thing to try it if you are concerned about very level (smoothly solid-colored) dyeing.
Find a good recipe for washing machine dyeing with Procion MX dyes. There are links to good recipes on my page "How can I dye clothing or fabric in the washing machine?". Top-loading washers are easier to dye in, but it is possible to use a front-loading washer for dyeing, usually with good results.
After you dye the sheets, having reset the machine repeatedly to get a good long time for the dye to penetrate and bond with the fabric (I always allow a full hour), you should wash the excess dye out, first with one washing of cool water, to get out the bulk of the excess dye, as well as the salt and the soda ash; then wash in hot water (again, this should be 140°F or higher) to remove the last of the unattached excess dye. This usually takes two full cycles through the washer, with hot water. First peer inside your washer and use a rag to wipe down any spots of dye that happened to splatter above the water line of the washing machine load. There should be no dye remaining in the machine when you are done washing out your sheets, but some dyers like to make sure by running a load of bleach, with the maximum load size, with towels or something, after they've completed the sheet washing-out. You can feel safe in machine drying your freshly dyed sheets, after you've completed the washing out steps.
I have to point out to you that there is often a bit of a scam involved in 1000-thread-count sheets, though it won't affect your dyeing at all. It's typically the case that a sheet labeled with a high thread count actually has a far lower thread count. The thread count is supposed to be the sum of how many threads are woven in one inch in the weft direction, plus how many threads are woven in the warp direction, so you might be expected about 500 threads per inch in reality (or possibly 300 one way and 700 the other). However, if you get a linen counter, which is a magnifier attached to a square of the correct dimensions, and count for yourself, you will commonly see a far lower count, perhaps 170 by 170. Manufacturers who know how much we love the idea of high thread-count sheets may fraudulently inflate their numbers by counting a thread as two or threads if it is composed of two or three finer threads twisted together, although that's a different thing than high thread count. See, for example, this article by Lauren Collins in the New Yorker magazine, "Splitting Threads", from their January 28, 2008 issue. Instead of believing the thread count claims made by a manufacturer, it is important to buy sheets by feel, based on whether they feel as soft and luxurious as you expect them to.
Good luck in dyeing your sheets. It's very satisfying to have the color you really want for your sheets, instead of one of the limited number of colors that the fashion industry has decreed will be available for sale this year. Don't neglect to consider the low water immersion process for your sheets, if you'd like a combination of colors in a very subtle pattern. Low water immersion dyeing is even easier than the washing machine method outlined above.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)