« 2011 July | Main | 2011 May »
Thursday, June 30, 2011
Before I go buying a bunch of table cloths that will not take dye, do you know if polyspun fabric can be dyed?




Tom Rolofson and Martine Purdy's


Advanced Tie Dye Techniques: Making Shapes and Mandalas
Message: Hi. In a moment of insanity, I had an idea that it would be cool to tie dye the table cloths for my son's upcoming wedding rehearsal dinner. Before I go buying a bunch of table cloths that will not take dye, do you know if polyspun fabric can be dyed? You know, the type of fabric found in your average restaurant on the table. (From a restaurant supply place). If so, is there a dye brand you would recommend? Thank you.
Wednesday, June 29, 2011
Where can I get some dye fixative, fast?




Tom Rolofson and Martine Purdy's


Advanced Tie Dye Techniques: Making Shapes and Mandalas
Country or region: USA
Message: Hi was told ritz dye worked good for tie dying and have the banded ones 1/2 soaking in the dye bath now. Then I read about the having to put something on it to keep it from bleeding out. I have called all our craft stores here and nobody has any. Where can I get some and fast? I was making these shirts for the 4th of July. Dang !
Sunday, June 26, 2011
What is the best way to wash several new tie dyed 100% cotton shirts to prevent color fading?
Country or region: USA
Message: What is the best way to wash several new tie dyed, 100 percent cotton shirts to prevent color fading? (tulip dye) Thank you!
Friday, June 24, 2011
Using fabric paint on a polyester crinoline slip
Message: Hi Paula,
 I want to paint this white crinoline slip blue, since I can't do the whole disperse dye thing and it's supposedly polyester. How much paint do I need to buy for the smallest size available? I'm trying to figure out if it will be more cost effective to use Dye Na Flow as opposed to a thicker fabric paint like Jones Tones or Dharma Pigment Dye which can be diluted a lot more. Shipping costs made me think twice about ordering online or by mail. Also, would you recommend sponge painting for a project like this? I don't mind a watercolor or cloud-like effect. Thank you so much!
I want to paint this white crinoline slip blue, since I can't do the whole disperse dye thing and it's supposedly polyester. How much paint do I need to buy for the smallest size available? I'm trying to figure out if it will be more cost effective to use Dye Na Flow as opposed to a thicker fabric paint like Jones Tones or Dharma Pigment Dye which can be diluted a lot more. Shipping costs made me think twice about ordering online or by mail. Also, would you recommend sponge painting for a project like this? I don't mind a watercolor or cloud-like effect. Thank you so much!
Thursday, June 23, 2011
What kind of dyes will work to change a cashmere cardigan from pink to black?
Country or region: UK


Message: Hi there,
I have a pink colour pure cashmere cardigan that I hate its colour. I want to dye it dark black. Do you sell washfast very dark black that after dyeing the cardigan doesn't fade? How much is it? I live in York, UK.
I will try not to change the temperature suddenly so that my cardigan doesn’t felt. Do you think it will shrink?
Wednesday, June 22, 2011
I'd like a link to follow your blog in my Google reader
Country or region: United States
Message: I'd like a link to follow your blog in my Google reader. I don't have Twitter. How can I do this?
(Please help support this web site. Thank you.)
Tuesday, June 21, 2011
Which is the best dye formulation to use for a nylon/elastane sports skirt?


Country or region: Greater Manchester, England
Message: I have a 80% Nylon and 20% Elastane sports blue/yellow piping skirt which I would like to dye all black. Which is the best dye formulation to use? Thanks, Liz
Monday, June 20, 2011
Dye safety and bladder cancer
Country or region: Canada
Message: Hi Paula,
I read your text about the chemical structure of red dye and was particularly interested that azoic dyes, which you recommend not using, are often referred to as "azo dyes."
I was just diagnosed with a very early stage of bladder cancer, and read that one of the risk factors is "exposure to chemicals....of particular risk are a type of dyes that include 'azo' compounds." (Here's the link to that statement.)
Do you think that this source means azoic dyes? How can I find out for sure? I'm an avid dyer who uses procion dyes, always wearing a dust mask when mixing the powders (which I don't do very often; I keep the liquid dyes refrigerated for at least a month), and always wearing gloves. I would be totally devastated if I had to give up dyeing, but I obviously don't want to increase my risk of a new tumor growing.
Anything you can do to help me figure this out would be greatly appreciated!
and the book,
Friday, June 10, 2011
I'm having trouble with dyeing plush. What can you recommend to dye this kind of fabric?
Country or region: Brazil - SP
Message: Hello, I am a fursuiter here in Brazil and I'm having trouble with dyeing plush. What can you recommend to dye this kind of fabric?
Currently I use airbrush and acrylic ink, but it comes off, with time.
There is another type of dye that does work on acrylic fibers, and in fact produces more intense colors on acrylic than disperse dye does. This dye is called basic dye, or cationic dye, because the dye molecule bears a positive electrical charge, and it includes the very first synthetic dyes that were invented. I usually recommend against the use of basic dye, because it is more dangerous to the user's health than many types of dye, and it is hard to work with because it stains everything you get it on, unlike the other dyes. In addition, it is more difficult to obtain than the safer disperse dyes. However, basic dyes are widely used in industry to dye acrylic clothing; if you have any acrylic socks, for example, which are not white, then they were dyed with basic dye. You can order modified basic dye, suitable for use on acrylic, from Aljo Mfg in the US; Aljo sells it for use as a silk paint, in their "alcohol/water dyes".
If you can't obtain disperse dye, or if your fabric will not tolerate boiling water, then your best solution may be the airbrushed acrylic paint that you are already using, in spite of your problems with its wearing off. Note that some acrylic paints should not be diluted much; if they are diluted with too much water, then they will not bind well to the underlying material, and will wear off much more quickly. Jacquard Products fabric paints, for example, which are based on an acrylic binder, must be diluted with no more than 25% water, by volume, for airbrushing. It's possible that you could get longer-lasting results from a different brand of acrylic paint than the one that you are currently using, or by diluting it less, if doing so does not make it too thick to use in the airbrush.
(Please help support this web site. Thank you.)
Thursday, June 09, 2011
Do you still have Dylon Run Away?
For more information, see "What chemicals can be used to remove dye?".
(Please help support this web site. Thank you.)
Wednesday, June 08, 2011
Would it be best to try to lighten the color of the cotton before trying to dye it, or to simply dye over the pink?
Country or region: USA
Message: I have a fairly bright, bubblegum-y pink cotton dress with white detailing and a polyester lining. I'd like to dye the dress purple. I understand that the polyester lining likely won't take color, the detailing will probably turn out a darker color, and that I should use a cold water fiber reactive dye for best results. Would it be best to try to lighten the color of the cotton before trying to dye it, or to simply dye over the pink?
My apologies if this has been asked already. I looked through previously asked questions and didn't find anything that I felt really answered my question. Any input you have will be greatly appreciated. Thank you in advance!
Tuesday, June 07, 2011
Will a different dye work to color both the clothing and its stitching?
Country or region: UK
Message: Hello,
I dyed a top using Rit all-purpose dye and all went well apart from stitches which darkened a little but stayed the same colour. Now I have almost identical top (the same brand and line so the stitching will be the same) and would like to know whether it is possible to solve this problem by getting a different type of dye?
(Please help support this web site. Thank you.)
Monday, June 06, 2011
Does batik fabric from India need to be washed before I use it in a quilt top?
Country or region: US
Message: Does batik fabric from India need to be washed before I use it in a quilt top?
Even for an unpieced quilt with a backing of the same color, you should pre-wash the fabric, I think, if only to pre-shrink it. I imagine that a whole cloth quilt, made without piecing at all, would get weirdly puckered later, if it shrinks after quilting. I would wash the fabric in hot water to be sure that it is pre-shrunk.
Sunday, June 05, 2011
I think it would be a good idea to soak the lace in a chemical that will neutralize any remaining hypochlorite from the bleach. The most convenient one to use is hydrogen peroxide, the 3% dilution that is sold at the pharmacy for use as an antiseptic. Soak the lace in the peroxide (straight or diluted with a quart or two of water) for ten minutes or so, then rinse and wash as usual.
If you happen to have Anti-Chlor (sodium metabisulfite, used in brewing wine and beer at home) or Bleach Stop (sodium thiosulfate), those work very well for this purpose, too. Peroxide is just as good and is easier to obtain locally in a hurry. I do not recommend the use of an acid, such as vinegar.
For more information, please see my page, "How can I neutralize the damaging effects of chlorine bleach?".
(Please help support this web site. Thank you.)
Friday, June 03, 2011
Can you suggest a monofunctinal dye that would give a darker shade than Reactive Blue 19? Name: Marlin


Victor B. Ivanov's

Reactive Dyes in Biology and Medicine
Explains use of reactive dyes for staining proteins or carbohydrates


Waring and Hallas's


The Chemistry and Application of Dyes (Topics in Applied Chemistry)
includes recipes for synthesizing reactive dyes


John Shore's

Cellulosies Dyeing
Useful information about the chemistry of reactive dyes, and other dye types


Country or region: USA
Thursday, June 02, 2011
Why do some dye color mixtures bleed a different color around the edges?
Country or region: USA
Message: Hi, I have been dyeing for several years now and have been working on mixing my own colors for a little over a year now. I am trying to figure out why some colors I mix will bleed or leach another color—like my browns always have green or yellow around them, and some reds have yellow run from them, for a few examples. Have you ever heard anything like this? Could I be doing something wrong? Any suggestions you have would be greatly appreciated.
Wednesday, June 01, 2011
Can you suggest a monofunctional reactive black dye? Name: Marlin


Victor B. Ivanov's

Reactive Dyes in Biology and Medicine
Explains use of reactive dyes for staining proteins or carbohydrates


Waring and Hallas's


The Chemistry and Application of Dyes (Topics in Applied Chemistry)
includes recipes for synthesizing reactive dyes


John Shore's

Cellulosies Dyeing
Useful information about the chemistry of reactive dyes, and other dye types


Country or region: USA
Is there any particular reason why another color, such as navy blue, will not do? There are a great many more reactive navy blue dyes. There is even a little bit of overlap in names or colors between some of the reactive blacks and some of the reactive dark blues. In fact, reactive black 5 itself has a distinctly bluish cast.
Here's a list of the unmixed reactive black dyes for which I have some information, in order of their Colour Index names:
1. reactive black 1, a monochlorotriazine dye (Procion Black H-G). Appears to be a chromium-cobalt complex.
2. reactive black 5, a vinyl sulfone (Remazol Black B). Bifunctional.
3. reactive black 8, a monochlorotriazine (Procion H-N). Appears to be a chromium-cobalt complex.
4. reactive black 13 (Cibacron Grey G-E).
5. reactive black 23 (Cibacron Pront Grey 2 B).
6. reactive black 26, a methylsulfonyl dye (Levafix Black P-G).
7. reactive black 31, a vinyl sulfone (Remazol Black RL). Looks bifunctional.
8. reactive black 39, a monochlorotriazine (Procion H Blue P2R). Looks monofunctional.
9. reactive black 49. (Dycrofix Blue P3R). Looks monofunctional. Actually reactive blue 49.
10. reactive black 160, monochlorotriazine (Black HEBL). Looks bifunctional. Actually reactive blue 160.
There is no reason to believe that my list is complete, though these are the most likely to be locatable.
More details on the above list:
1. Reactive Black 1, Procion Black H-G. CAS Number: 12236-77-0. Molecular Formula: C23H13ClN8Na2O10S2 [this formula omits the chromium and cobalt]. Synonyms:
1-Naphthalenesulfonicacid,4-[[6-[(4-amino-6-chloro-1,3,5-triazin-2-yl)amino]-1-hydroxy-3-sulfo-2-naphthalenyl]azo]-3-hydroxy-7-nitro-, chromium-cobalt complex (9CI); C.I. 17916; Cibacron Black BG; Cibacron Black BG-A; Cibacron Black FBG-A; Cibracron Black BG-A; Procion Black H-G. Available from Sigma Aldritch as Cibacron Black FBG-A.
2. Reactive Black 5, which you've already rejected for its bifunctionality, is the easiest-to-find reactive black dye (that is not a mixture of other colors). It is symmetrical with its two reactive ends, as shown in the molecular structure drawing below.
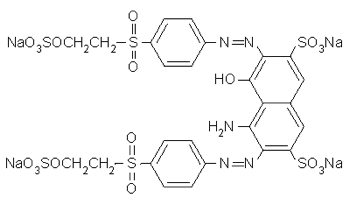
Its full chemical name is [2,7-naphthalenedisulfonic acid, 4-amino-5- hydroxy-3,6-bis((4-((2-(sulfooxy)ethyl)sulfonyl)phenyl)azo)-tetrasodium salt], while its formula is C26H21N5Na4O19S6, CAS Reg. No. 17095-24-8. The formula will be useful below for comparing to a dye for which little other information is available.
3. Reactive Black 8, also known as Black DN or Black HN, is a monofunctional black reactive dye, in the monochlorotriazine (Procion H) group, but, like Reactive Black 1, it is described as occurring in a 1:2 chromium-cobalt complex of two dye molecules per metal atom. When complexed to chromium alone, the dye produces blue, while when complexed to cobalt alone, it is reddish. Synonyms: C.I. 18207; Cobalt 5-[(4-amino-6-chloro-1,3,5-triazin-2-yl)amino]-4-hydroxy-3-[(2-hydroxy-5-nitrophenyl)azo]-2,7-naphthalenedisulfonic acid complex; Basilen Black P-BR; Cibacron Black B-D; Drimarene Black P-BL; Helaktyn Black DN; Kayacion Black P-N; Ostazin Black H-N; Procion Black H-N; Procion Black P-N; Reactive Black K-BR; Reactive Black MN. CAS Registry Number 12225-26-2.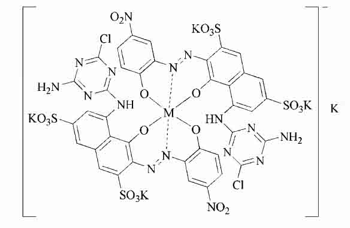
[picture from Industrial dyes: chemistry, properties, applications, by Klaus Hunger, p. 312 (John Wiley and Sons, 2003).]
I have another picture of Reactive Black 8; unfortunately I cannot determine its source. What's interesting about it is that it contains essentially the same dye molecule as the picture above, but with no mention of the chromium or cobalt, nor of a complex of two or more dye molecules:
The fact that a dye takes the form of a dimer complexed with chromium and/or cobalt makes little difference to most purposes, but might be a real problem in cases in which only a monofunctional dye will do. Having seen these two different drawings for what is essentially the same dye, I think we may need to wonder about whether any single monofunctional dye is involved in a similar complex.
4. Reactive Black 13. Cibacron Grey G-E; Hicion Grey HE-G; CAS 12225-31-9. I have no more information on this dye. Sigma Aldritch carries it; contact them for more information.
5. Reactive Black 23. Cibacron Pront Grey 2 B. I have no more information on this dye. Sigma Aldritch carries it; contact them for more information.
6. Reactive Black 26. Levafix Black P-G; Levafix Black E-2G. CAS 12731-61-2. I have no more information on this dye.
7. Reactive Black 31 is listed as being in the Remazol series. Synonym: Black RL. CAS# 12731-63-4
I do not have the molecular structure of the dye reactive black 31, but I have a molecular formula:
C29H20N6O17S4•4Na
Comparing that to the molecular formula of reactive black 5, C26H21N5Na4O19S6, the two look similar in size, which leads me to suspect that reactive black 31 may also be a bifunctional dye.
It is carried by Jagson and Shivitex; the latter provides fastness ratings for this dye.
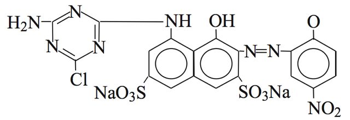
8. Reactive black 39 is in the Procion H series, sold by Jagson as Dycrofix Blue P2R (their color chip shows a pale gray). Synonyms: Jagson's Dycrofix Blue P2R; Ostazin Blue H-2G; possibly Cibacron Navy G.
Picture source: Environmental chemistry of dyes and pigments, Abraham Reife, H. S. Freeman, page 12 (Wiley-Interscience, 1996).
9. Reactive black 49. This appears to be a misprint for Reactive Blue 49. Jagson shows Reactive Blue 49 with a black color chip, so it's worth considering in spite of its name.
Synonyms: 2-Anthracenesulfonicacid,1-amino-4-[[3-[[4-chloro-6-[(3-sulfophenyl)amino]-1,3,5-triazin-2-yl]amino]-2,4,6-trimethyl-5-sulfophenyl]amino]-9,10-dihydro-9,10-dioxo-,sodium salt (1:3); Cibacron Blue 3R; Cibacron Blue P 3R; Cibacron Brilliant Blue 3R-P; Helaktyn Blue D 3R; Kayacion Blue P 3R; Levafix Blue PN 3R; ProcionBlue P 3R; Procion Brilliant Blue H 3R;. CAS 72927-99-2. Molecular Formula: C32H26 Cl N7 O11 S3 • 3 Na
Structure from http://www.lookchem.com/cas-729/72927-99-2.html. It looks like a Procion H type (monochlorotriazine).
10. Reactive black 160 is sold by Jagson as Black HEBL, with a medium gray color chip. (See http://www.jagson.com/reactive_dyes.htm). They don't give any other identifying numbers. Chemicalregister.com lists a reactive black 160, but it appears to be a misprint for reactive blue 160. Here's their image for reactive black 160:
Here's an image of reactive blue 160, from guidechem.com, which is easier to read but does appear to be the same molecule: you can see two aminochlorotrizine (monochlorotriazine) groups in it.
Before I go buying a bunch of table cloths that will not take dye, do you know if polyspun fabric can be dyed?
Name: Jo
—ADVERTISEMENTS—
Tom Rolofson and Martine Purdy's

Advanced Tie Dye Techniques: Making Shapes and Mandalas
Country or region: USA
Message: Hi. In a moment of insanity, I had an idea that it would be cool to tie dye the table cloths for my son's upcoming wedding rehearsal dinner. Before I go buying a bunch of table cloths that will not take dye, do you know if polyspun fabric can be dyed? You know, the type of fabric found in your average restaurant on the table. (From a restaurant supply place). If so, is there a dye brand you would recommend? Thank you.
No, you really don't want to dye polyester tablecloths! You'd need to buy an enormous cooking pot to boil each one for an hour in a special polyester dye, plus a horribly smelly carrier chemical. It would take a five-gallon pot to dye even a moderately sized tablecloth, so the cost would be prohibitive. The effort would be far more than it's worth. It's so much better to chose an easily dyeable fabric. You've probably guessed as much already, since you wrote to ask.
Cotton tablecloths would be far easier to dye, since you could use fiber reactive dyes at room temperature. Not just any cotton tablecloth will do, however. Even if you find tablecloths made of an easily dyeable fiber, there may still be a serious problem. If there is a stain-resistant finish on a tablecloth, it will resist dye as well as stains, preventing the dye from reaching the fabric. Wrinkle-resistant treatments interfere with dyeing, too, though not as badly.
It's not easy to find dyeable tablecloths. Dharma Trading Company sells some, in three sizes: 52 inches square, or 60 inches by 72 inches, or 60 inches by 102 inches. Note that those measurements are before washing, though, and significant shrinkage is to be expected. Find out what measurements you will need before deciding whether this project is doable.
Williams Sonoma sells white cotton hotel tablecloths with no claims of stain-resistance or wrinkle resistance; customer reviews make it clear that the tablecloths must be ironed, preferably with starch. This suggests that they might be dyeable. You would need to dye just one first, as a test, before knowing whether or not it would work; of course, you could not return your test tablecloth, once it is dyed, but if you kept the rest of them in their packaging until after your test, you could return them, if your test does not turn out well.
Note that only the Dharma tablecloths are labeled as having cotton thread. If a tablecloth is not labeled as to its thread content, you can be sure that the thread used in sewing the hem is made of polyester, which will not dye, but will instead remain white. This is okay in tie-dyeing, but might look odd if you use a dark color to dye the tablecloths a solid color.
Another option for you would be to use polyester tablecloths in any commercially available color, and top them with a dyeable table runner.
After you have purchased dyeable cotton or linen tablecloths, and prewashed them, I recommend that you dye them with fiber reactive dyes, such as Procion MX dyes. It's very easy to do, because these dyes are set with washing soda, rather than with heat. The tie-dyeing kits sold by Dharma Trading Company are an excellent choice. Consider whether you want to tie-dye your tablecloths, or use low water immersion dyeing, or dye them solid colors in the washing machine. You can find instructions for these and other dyeing techniques in the instructions section of my web site, at http://www.pburch.net/dyeing/instructions.shtml.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Wednesday, June 29, 2011
Where can I get some dye fixative, fast?
Name: Di
—ADVERTISEMENTS—
Tom Rolofson and Martine Purdy's

Advanced Tie Dye Techniques: Making Shapes and Mandalas
Country or region: USA
Message: Hi was told ritz dye worked good for tie dying and have the banded ones 1/2 soaking in the dye bath now. Then I read about the having to put something on it to keep it from bleeding out. I have called all our craft stores here and nobody has any. Where can I get some and fast? I was making these shirts for the 4th of July. Dang !
It would be easier to wash those shirts now in hot water, to get the Rit dye out, and then start over on them with a better dye. Unfortunately, all-purpose dye, such as Rit dye, is really not a good dye for tie-dyeing.
The best way to quickly get the materials you need to make high-quality tie-dyes, with the minimum of work, would be to call those same crafts stores, or a sewing store, and find one that sells a good tie-dyeing kit. Look for the Jacquard tie-dyeing kit, or a smaller kit made by the same manufacturer that is called the Funky Groovy Tie-Dye Kit. If you can't find those, look for a kit made by Tulip, such as the Tulip Ultimate Tie-Dye Kit or the Tulip One Step Tie-Dye Kit. All of these kits contain high-quality fiber reactive dyes, which will stay bright and vibrant for years. Avoid the Rit tie-dye kit.
Even if you could find the right dye fixative for all-purpose dye locally, the fixative will not make it suitable for multi-colored tie-dyeing. All-purpose dyes run easily in the wash. The first time you wash the shirts (which you must do to prevent loose dye from rubbing off), bits of the dye dissolve in the water and are then redeposited on other parts of the fabric. As a result, the colors become dull very quickly. The commercial dye fixative Retayne, and similar products, will work to stop all-purpose dye from fading so quickly in the laundry, but they cannot keep the dyes vibrant and unmuddied in the first place. For that, you need to use a fiber reactive dye, such as the Procion dyes that are used in all the good tie-dye kits.
Over the years, I have received many sad emails from people who got bad results from trying to tie-dye with all-purpose dye. The colors are dull and fade quickly. When these same people try using a higher quality dye, such as the ones in the Jacquard or Tulip kits, they write about how amazingly better their tie-dyes look. Using the right materials makes a huge difference in how attractive the tie-dye shirts you make will turn out to be. It's really not worth the effort to try to tie-dye with inferior dyes, when you could just go to a craft or sewing store and buy the high-quality type of dye.
All-purpose dye works better for a single color tie-dye. If you tie circles or bulls-eyes into a shirt, you can drop it into a pot of boiling Rit dye and get pretty reasonable results, especially if you manage to buy some Retayne from a quilting supply shop or online to stop the dye from running. When you use only a single color, you don't have to worry about the colors running together and turning muddy. This is the original type of tie-dye that has been done for hundreds of years. Multi-colored tie-dye did not become practical until better dyes were introduced in the 1960s and 1970s.
Whatever you do, don't try to use all-purpose dye, such as Rit dye, at room temperature. All-purpose dye is a hot water dye: it must be used in water that is simmering on the stovetop in order to work at all. Soaking at room temperature does not work. The dye will mostly wash out in the laundry.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Sunday, June 26, 2011
What is the best way to wash several new tie dyed 100% cotton shirts to prevent color fading?
Name: Connie
Country or region: USA
Message: What is the best way to wash several new tie dyed, 100 percent cotton shirts to prevent color fading? (tulip dye) Thank you!
This is the washing technique for tie-dyed t-shirts that have been dyed with fiber reactive dyes (such as Procion dye) and soda ash, after you've allowed the shirts plenty of time to react with the dye, preferably overnight in a warm place (70°F or warmer):
- Wash once in cool (or room temperature) water, to remove the soda ash, urea, and salt, and some of the extra dye, then wash at least twice in very hot water. The hotter the water is, the more efficiently it will remove the unattached excess dye. Soaking works best. If water is in short supply, use a cheap cooler (not to be reused for food) to soak your dyed fabrics in hot water.
Some dyers even use boiling water, for greatest efficiency in removing the unattached dye. Unlike other types of dye, fiber reactive dyes retain their strong bond to the fabric even in boiling water.
In your initial cool wash, either avoid detergents (since they may have a high pH), or use Synthrapol or its generic equivalent. In the later washes, use Synthrapol if you've got it, or any laundry detergent if you don't.
It is much more important to use really hot water than it is to use Synthrapol. Sufficiently hot water will remove backstaining, if you have allowed plenty of extra dye reaction time before washing out. Allowing plenty of time is essential for making sure that all of the dye has fully reacted, before you move or untie your tie-dyes; this prevents permanent backstaining that cannot be removed.
Always start with a single cool water wash, though, to reduce the tendency of the unbound dye to form loose Rit-dye-like associations with the fabric, which are more trouble to wash out. This is more of a problem if your dyes are very old, so that you have to use more to get the same amount of reactive dye activity.
Although Tulip tie-dye kits are claimed to include all of the chemicals you need, already mixed into the dye powder, when you buy them, the Tulip Dye company says that best color results can be obtained by presoaking your items in soda ash, dissolved in water. This might imply that they do not include as much soda ash in their kits as they should. It won't make any difference to the proper way of washing out the excess dye after dyeing, though.
If your tie-dyes made with reactive dyes fade when you use the hot water method to wash the excess dye out, the problem is not caused by the washing method, but by not using enough dye to begin with (it should be darker than you want, before washout), by not allowing enough reaction time (overnight or longer is best), by not keeping the clothing warm enough while the dye reacts with it (under 70°F or 21°C is a problem), or by letting the dye dry on the fabric too quickly (which can be prevented by using urea in the dye mixtures, by wrapping in plastic overnight, or by working in a place that has a humid environment). See the FAQ, "My colors just washed out! What happened?". I have heard more than once that the colors produced by the Tulip kits are not as vibrant as those from other brands of tie-dye kits, such as from Jacquard Products or Dharma Trading Company, although they do contain the same good type of dye.
Note that tie-dyes made with all-purpose dye, such as Rit dye, will bleed badly when washed, creating big problems for multi-color tie-dye designs. Anything that is dyed with all-purpose dye must never be washed according to the instructions I gave above! It must be washed only in cold water, by hand, separately from other items. Don't ever follow the hot-water reactive-dye washing-out technique with all-purpose dyes, direct dyes, or acid dyes.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Friday, June 24, 2011
Using fabric paint on a polyester crinoline slip
Name: Lunia
Country or region: US
Message: Hi Paula,
 I want to paint this white crinoline slip blue, since I can't do the whole disperse dye thing and it's supposedly polyester. How much paint do I need to buy for the smallest size available? I'm trying to figure out if it will be more cost effective to use Dye Na Flow as opposed to a thicker fabric paint like Jones Tones or Dharma Pigment Dye which can be diluted a lot more. Shipping costs made me think twice about ordering online or by mail. Also, would you recommend sponge painting for a project like this? I don't mind a watercolor or cloud-like effect. Thank you so much!
I want to paint this white crinoline slip blue, since I can't do the whole disperse dye thing and it's supposedly polyester. How much paint do I need to buy for the smallest size available? I'm trying to figure out if it will be more cost effective to use Dye Na Flow as opposed to a thicker fabric paint like Jones Tones or Dharma Pigment Dye which can be diluted a lot more. Shipping costs made me think twice about ordering online or by mail. Also, would you recommend sponge painting for a project like this? I don't mind a watercolor or cloud-like effect. Thank you so much!Your willingness to go for a watercolor or cloud-like effect makes this a completely reasonable project. A mottled blue could be very pretty on your crinoline slip. Even the most amateur effort would show to advantage peeping out under a dress that covers most of it. It would be particularly beautiful to combine two or more colors.
I do need to point out that this would not be a good project if you wanted a single solid color, since fabric paint does not give a perfectly smooth solid color, the way dye does, but instead tends to produce somewhat mottled results. I get letters now and then from people who want to use fabric paint to color formal dresses a perfectly smooth solid color for a wedding, and I have to tell them not to bother.
It's important to know that the thicker type of fabric paints CANNOT be diluted more than thinner paints. Jones Tones and other thicker paints cannot be diluted to the point of being thin, because then there will not be enough binder present to glue the pigments to the fabric. A thicker fabric paint inevitably leaves a more noticeable feeling on the fabric than the thinner paint does; you cannot dilute it more to make it thinner, and still have it cover the original white color well, and stick well to the fabric.
The best way to pigment dye a polyester garment like this might be to crumple it up into a relatively small container, dampen it with water, and submerge it in fabric paint that has been diluted to the maximum allowed in the manufacturers' instructions, squeezing the paint throughout, and then hang the garment up to dry, carefully pulling at it as much as you need to in order to restore its original shape. You don't want the slight stiffness of the paint to interfere with the way the fabric lies. As it dries, the paint will become darker at the seams, where the paint tends to accumulate. Diluting the paint more than the manufacturers advise can cause the paint to cover less well and wear off more quickly. Perhaps wear is not an issue on a garment like this, though, which may be worn only once, so it might be reasonable to dilute it more. That slip looks as though it will be difficult to compress very much.
Alternatively, sponge-painting is a good way to do it. You could hang it on a non-rustable clothes hanger, over a waterproof tarp (preferably working out-of-doors), or lay it out flat on the tarp and do first one side then the other. Dampen the entire garment, and apply paint with a sponge or a large brush. If you do this, you must be careful to apply it quickly, applying wet-to-wet, not allowing any of it to dry before you have completed applying the paint. If you apply wet paint next to paint that has already dried, you will see a sharp line where the two applications of paint meet. As with the other application method, the paint will tend to accumulate more in the seams.
I think it's important to dampen the fabric before applying the paint. In fact, it might be best to soak it overnight in water, to make sure the water penetrates as much as possible, and then squeeze out as much of the water as possible immediately before applying the paint. This may be less important on polyester than on cotton or wool, though.
Do not use any fabric paint whose manufacturer does not recommend it for use on polyester. Some paints do not work on synthetic fibers. Jacquard Products says that all of their fabric paints will work on polyester.
How much paint do you need? Even the smallest size of that crinoline looks as though it is quite voluminous. I can't imagine that you'll need less than a quart of diluted paint. You might need considerably more. You will need to wash the garment before you paint it, to give the paint its best chance to adhere. Of course, you'll need to do this by hand. Pour water onto your crinoline using a one-quart pitcher, and use this to make a rough estimate of how much liquid it will take to color all of the material. I'd advise you to at least double this estimate when ordering your fabric paint. It is far better to have more fabric paint than you need, than less, in order to avoid letting some of the paint dry on the garment before you can acquire more.
Dharma Pigment Dyes are the most economical of the very thin fabric paints, because they can be diluted more. They are quite thin, like Dye-Na-Flow. One four-ounce bottle can be diluted to twenty fluid ounces (600 ml). Unfortunately, the colors are not currently available on the Dharma web site; perhaps it has been discontinued.
Jacquard Products' Dye-Na-Flow is a fabric paint that has been made very thin, to mimic the feel of a dye. You can dilute it only a little, by adding up to one-quarter its volume of water, though for an item that will not have to survive much wear you might be able to get away with adding more. One quart of Dye-Na-Flow lists for $34, or one gallon for $79, but you can probably find a lower price. I think Dharma sells quarts for $25, but I don't currently see it on their web site (perhaps there is a temporary malfunction there). To find a local retailer from which to buy Dye-Na-Flow, try the Jacquard Products Store Locator. The larger quart-sized jars of Dye-Na-Flow purchased via mail order may be more economical than the smallest jars at a local shop. (The gallon-sized jars are available by phone order from their Bulk & Specialty store.)
It's a pity that your crinoline slip happens to be made of polyester. The traditional fiber for crinolines was cotton. I used to use that same cotton crinoline to wipe metal plates in printmaking class. You can still buy stiff cotton crinoline fabric, which is much more easily dyeable than the polyester version, but it will not be inexpensive to find someone local to sew a new one in the shape that you want. Check out the beautiful hand-dyed mermaid crinolines being offered for sale on Etsy.com.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, June 23, 2011
What kind of dyes will work to change a cashmere cardigan from pink to black?
Name: Mary
Country or region: UK
—ADVERTISEMENTS—
Washfast Acid dyes
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!
Message: Hi there,
I have a pink colour pure cashmere cardigan that I hate its colour. I want to dye it dark black. Do you sell washfast very dark black that after dyeing the cardigan doesn't fade? How much is it? I live in York, UK.
I will try not to change the temperature suddenly so that my cardigan doesn’t felt. Do you think it will shrink?
There's a very real risk that your cashmere cardigan will shrink when you dye it. Being careful to avoid agitation and sudden temperature changes will help, but it won't guarantee that the sweater will still fit you after you dye it. Because of this risk, I recommend that you try dyeing this sweater only if you would otherwise discard it.
I don't think you should purchase dye through the affiliate links on my site, because they are through US companies only. If you look under the "Europe" subheading on my page, Sources for Dyeing Supplies Around the World, you will see several different dye suppliers that are located in the UK, which would be more suitable sources for you. If you do choose to order dyes from the US, PRO Chemical & Dye has the widest range of different kinds of dyes for wool, and they do ship to the UK. Kraftkolour in Australia has an even wider variety of dyes, but higher prices.
There are several different types of acid dyes that are suitable for hand-dyeing cashmere. The most washfast (resistant to fading in the laundry) are from a category of acid dyes which is variously known as premetallised acid dyes, premetalized acid dyes, or metal complex dyes. Another type of acid dyes, the acid milling dyes, is less washfast than premetalized acid dyes, but much more washfast than acid leveling dyes. The less washfast types of acid dyes can be satisfactory if you either always dry-clean the item, or launder it only by hand, in cool water.
In the UK, a good source for acid dyes for hand dyeing is George Weil; their acid dyes are probably of the acid milling type. Another good UK source of dyes in suitably small quantities is Kemtex. See my page of dye suppliers for contact information for these and other UK sources for acid dyes.
Lanaset Jet Black is a very satisfactory blend of two premetalized acid dyes, but it's difficult to obtain in the UK. (See my earlier blog post, answering "Do you know of a supplier for Lanaset dyes in the UK?".) ProChem's Wash Fast Black is also very satisfactory, as it contains one of the two premetalized acid dyes found in the Lanaset Jet Black. (See my Dye Forum post, "Lanaset Jet Black contains Washfast Acid Jet Black WF672 plus another black dye".) Kraftkolour, in Australia, is a good source of both premetallised dyes and Lanaset dyes.
Kraftkolour also sells Lanasol reactive dyes for wool, which can be used at a lower temperature, with an additive that they call "Cold Wool S". The low temperature would be very desirable for reducing the risk of shrinkage and felting, and they are highly washfast, even when laundered under harsh conditions. See their instruction sheet, "Cold dyeing system for wool and silk & other protein fibres" [PDF]. These dyes react with the proteins in wool fibers, just as Procion MX and other reactive dyes react with cotton and silk, but they do not require the high alkaline pH that Procion dyes require, in order to properly react, so they are far more suitable for wool. (Procion dyes can also be used with a low acid pH, instead, by substituting vinegar or citric acid for the soda ash normally used with them, but under those conditions they act as acid dyes, not as reactive dyes, so that the chemical bonds holding them to the fiber are significantly less strong.)
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Wednesday, June 22, 2011
I'd like a link to follow your blog in my Google reader
Name: Judy
Country or region: United States
Message: I'd like a link to follow your blog in my Google reader. I don't have Twitter. How can I do this?
I just tried Google Reader for the first time to test my answer to your question, and I'm happy to see that it works. Add a subscription to this URL: http://www.pburch.net/dyeing/dyeblog/rss.xml
This link is listed on the blog page itself, but it's not very obvious. It's in the right margin of the blog, about a page down, under the subheading "XML/RSS FEED". The link itself is labeled "Syndicate this site". One way to get the link is to right-click on it with your mouse to get a menu with an option like "Copy Link", then paste it into your Google Reader's "Add a Subscription" popup.
Only the titles of the blog entries show within Google Reader; when you click on the title, the blog page itself will open in your web browser.
In addition, using a web browser, you can look at the following URL to see the Twitter feed for the site, which includes the titles of new blog entries plus additional pointers to interesting forum discussions and new or updated pages: http://twitter.com/#!/handdyeing. You do not have to have a Twitter account in order to use this page.
The same updates are found in the Facebook page for the site, as well, which is located at http://www.facebook.com/handdyeing. You can view this page in your web browser without logging in to Facebook. I've been experimenting with using a Facebook plugin for the "What's New" display on the front page of the All About Hand Dyeing web site, at http://www.pburch.net/dyeing.shtml. The most recent entries, shown in the plugin, work even for people who have never logged in to Facebook, but it's a little annoying how Facebook slightly changes the way the plugin works now and then, without notice.
(Please help support this web site. Thank you.)
Tuesday, June 21, 2011
Which is the best dye formulation to use for a nylon/elastane sports skirt?
Name: Liz
—ADVERTISEMENTS—
Washfast Acid dyes
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!
at Paradise Fibers

Washfast Acid dyes
Also known as Nylomine dyes, excellent for use on nylon. One ounce of dye will dye six pounds of fiber!
Country or region: Greater Manchester, England
Message: I have a 80% Nylon and 20% Elastane sports blue/yellow piping skirt which I would like to dye all black. Which is the best dye formulation to use? Thanks, Liz
Nylon should be dyed with acid dyes. See my page on "How to dye nylon or polyamide".
Unfortunately, nylon requires heat in order to bond well to acid dyes, but elastane is very sensitive to heat. See my page, "How to Dye Spandex (also known as Lycra® or elastane)". You will have to use a lower-than-ideal temperature to dye the nylon, in order to avoid deforming and ruining the shape of the elastane in the skirt. Use the hottest water permitted in the care instructions included in the skirt; do not exceed 60°C (140°F). You will need to use a mild acid such as vinegar, carefully following the instructions provided with your dye.
Note that some nylons have a surface finish on them that will prevent dye from reaching inside the fibers, making dyeing impossible. It's difficult to predict whether the nylon in your skirt may have this problem. Dye-repelling finishes include stain resistance, permanent press finishes, 'performance' wicking finishes, and anti-pilling finishes, among others. Some nylons simply cannot be dyed satisfactorily.
It's possible that the piping in your skirt is not made of nylon at all. The fiber content of "trim" is frequently left off of garment labels. If the piping is made of polyester, you will not be able to dye it, because dyeing polyester requires extremely high heat, which will certainly damage the elastane in your skirt. Polyester will remain the original color after being dyed with most dyes.
If there is no problem with a surface finish, and if they are both made of the same fiber, the yellow and blue in your skirt will both be black, after you dye them with a large amount of black dye. However, they are unlikely to be the same shade of black. This is because all dyes are transparent, and always show through a little of the underlying color. The piping will turn from yellow to a greenish black, while the fabric will turn from blue to a bluish black. Using a lot of dye powder will help, to get the darkest black possible. You can repeat the entire dye process, using more dye powder, if the skirt is not a dark enough black after the first time. Using a temperature lower than nylon's ideal 85°C (185°F), so that you don't distort the spandex, may make it impossible to obtain the blackest black.
You can find acid dyes to buy in the UK at several different online retailers, such as Kemtex Educational Supplies or George Weil. See "Sources for Dyeing Supplies Around the World"; scroll down to the section of dye retailers in Europe.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Monday, June 20, 2011
Dye safety and bladder cancer
Name: Ann
Country or region: Canada
Message: Hi Paula,
I read your text about the chemical structure of red dye and was particularly interested that azoic dyes, which you recommend not using, are often referred to as "azo dyes."
I was just diagnosed with a very early stage of bladder cancer, and read that one of the risk factors is "exposure to chemicals....of particular risk are a type of dyes that include 'azo' compounds." (Here's the link to that statement.)
Do you think that this source means azoic dyes? How can I find out for sure? I'm an avid dyer who uses procion dyes, always wearing a dust mask when mixing the powders (which I don't do very often; I keep the liquid dyes refrigerated for at least a month), and always wearing gloves. I would be totally devastated if I had to give up dyeing, but I obviously don't want to increase my risk of a new tumor growing.
Anything you can do to help me figure this out would be greatly appreciated!
I'm sorry you've been the recipient of such vague and frightening information, on top of having to deal with early-stage bladder cancer itself. There are some dyes that can cause bladder cancer, but that does not mean that all synthetic dyes are dangerous, especially if used correctly, with appropriate safety measures.
The specific dyes that you are using, combined with the safety measures that you are using, are extremely unlikely to increase your risk of any cancer at all. Not only are you not using any of the dyes that have been linked to bladder cancer, but you are using quite small quantities of dyes, compared to employees in the dye industry, and you are taking suitable measures to minimize exposure to even those small amounts that you do work with. I do not believe that your past use of Procion dyes has contributed to your recent diagnosis.
Your information page includes this statement:
"Exposure to chemicals — Being exposed to certain chemicals or industrial compounds in the workplace or the environment can significantly increase the risk of bladder cancer. Of particular risk are a type of dyes that include "azo" compounds. In most cases, it takes many years after the chemical exposure for the person to develop bladder cancer."
This is rather infuriatingly vague. There are many dyes that contain azo linkages, but only some of them are thought to cause cancer. Azo means nothing more than that the dye molecule contains two nitrogens bonded to one another with a double bond, and carbons attached to each nitrogen. There are safe chemicals that contain azo linkages, and there are toxic and unsafe chemicals that contain azo linkages. It is not the azo linkage that makes a dye dangerous.
Some azo dyes are considered to be safe enough even to eat. Azo dyes that are permitted as food additives in Canada (since that's where you live) include tartrazine, sunset yellow, amaranth (not to be confused with the plant of the same name), and allura red. While I am not convinced that regularly consuming large amounts of artificial food dye is a good idea, the safety testing that has been performed with these dyes does prove that not all azo dyes are equally suspect.
The azo dyes that are suspected of causing cancer are based on the chemicals benzidine, o-tolidine, and o-dianisidine. Certain dyes are manufactured from these carcinogenic chemicals; the metabolism of these dyes by the body can produce these chemicals again. None of the fiber reactive dyes whose use I recommend are based on any of these dyes. In fact, no reactive dye at all is listed among the dyes based on these three chemicals. However, there are many direct dyes, including some that used to be contained in all-purpose dyes that were sold for home use, that are based upon them, and a few acid dyes, as well.
All-purpose dyes, such as Rit All-Purpose Dye and Tintex Fabric Dye, contain a blend of direct dyes and acid dyes. At one time, up to and including the 1970s, these household dyes include many dyes based upon benzidine or o-dianisidine. I think that it is probable that Rit dye no longer contains any of these dangerous direct dyes, only safer ones. Unfortunately, it's hard to be be certain, because the Rit dye company, and other companies that produce all-purpose dyes, maintains a high level of secrecy about which specific dyes are used in their products. I know that one dye based on o-dianisidine is currently being marketed by another manufacturer for children to use, in the form of "Tie Dye Cords", which are to be tied around fabric and then dropped into boiling water. Tie Dye Cords are strings that are impregnated with direct dyes. As a rule, Tie Dye Cords are not used by serious hand dyers, because the results are very much inferior to other dyes. (See "o-Dianisidine in dye-impregnated tie-dye cords" .)
The dyes that you, and all of us, are supposed to specifically avoid include those on this list: direct black 1, direct red 28, direct black 38, direct blue 6, direct green 6, direct brown 95, direct brown 2, direct blue 2, and direct black 4, and acid orange 45, acid red 85, acid red 114, acid red 167, acid black 209. (None of these dyes are included among the dyes listed on my WashFast Acid Dyes page, Leveling Acid Dyes page, or the other acid dyes on my lightfastness page.) A more complete list is in the appendix to the 1980 NIOSH alert, "Health Hazard Alert--Benzidine-, o-Tolidine-, and o-Dianisidine- Based Dyes", or in the rather lengthy post "Specific dyes to avoid", which is part of the Dye Forum discussion about tie dye cords that was linked in the preceding paragraph. If you are not using all-purpose dyes or direct dyes, then you are not using any of the dyes from this list. If you do use all-purpose dyes, I recommend that you (and everyone else who is concerned) switch to dyes whose specific identity is revealed by the seller, just so that you can be sure. There are some direct dyes whose sellers, unlike Rit, Tintex, Deka, or Cushing, will tell you exactly which dyes they contain. Although the dyes used in any particular premixed dye color are a trade secret, you can find which acid dyes or direct dyes are being used in the entire dye line when you buy from PRO Chemical & Dye, and which acid dyes are being used in the unmixed dyes from Jacquard Products or the new line of Dharma Acid Dyes. Knowing what dyes you are using, you can look up the MSDS and other safety information and be sure that they are not among the dyes you've been told not to use. You may find it simpler to just stick to fiber reactive dyes, such as the Procion MX dyes.
Naphthol or napthol dyes, which are sometimes also called azoic dyes, are hazardous in a different way. (See "About Naphthol Dyes".) Although some naphthol dyes are based on benzidine, o-tolidine, or o-dianisidine, there are additional hazards of this class of dyes that are not related to these chemicals. The worst thing about naphthol dye components is that some of them can be absorbed directly through intact skin, unlike reactive dyes which tend to react with the first skin cells they encounter and are thus less likely to proceed further into the body. A minor hole in a glove that would probably be no big deal with fiber reactive dyes is a serious matter with naphthol dyes, some of which are carcinogenic or mutagenic or possibly even teratogenic. However, this is not likely to be an issue for you, simply because I doubt you have ever considered using naphthol dyes. I don't know any hand dyers in North America who use naphthol dyes, though there are some in Australia and Indonesia. Some naphthol dyes are used in the textile industry in the US, but they are not sold by any hand dyeing supplier in the US or Canada. As long as you are buying dyes from a hand-dyeing supplier in Canada or the US, such as Maiwa, G&S Dyes, Dharma Trading Company, or PRO Chemical & Dye, you do not have to think about naphthol dyes at all.
Among hand dyers, anyone who is careful is at much lower risk than those who are exposed to high amounts of hazardous chemicals through their jobs. In their discussion of the causes of bladder cancer, the web site eMedicineHealth says, "Strict workplace protections can prevent much of the exposure that is believed to cause cancer." The care that you have taken to follow the rules of good dye usage is similar. Some dyers are careless with their dyes. Some dyers refuse to wear gloves at all, and dip their hands directly into their dyebaths; because they are using Rit Dye, and because Rit Dye is sold in the grocery stores without much in the way of warning labels, they assume that the dye is totally harmless. (See "Why We Should Be Very Careful When Using Chlorine Bleach", for one example.) The level of exposure among these careless dyers is vastly higher than that among people who follow normal precautions, such as wearing gloves to handle dye powders and dye solutions, wearing a dust mask when working with dye powders, and immediately wiping up spilled dyes. It's rather horrifying to think about home dyers in the fifties, sixties, and seventies dipping their hands into benzidine- or dianisidine-based dyes with no gloves or other protection, without any concern for risks. I am also concerned about the risks posed by tattoo inks, which are known to contain carcinogenic chemicals such as aromatic hydrocarbons, which are considered to be a risk for bladder cancer, and yet are injected into the skin, without regulation or disclosure of ingredients.
For further reading about dangerous dyes, see the following publications from the USD Centers for Disease Control :
• "Public Health Statement for Benzidine"and the book,
• The Artist's Complete Health and Safety Guide by Monona Rossol
Here's an interesting link to a short piece in the July 1982 issue of Mother Jones magazine about the dangers of benzidine-based dyes in Rit dye.
It is, of course, wise to avoid unnecessary exposure to any household chemical; I would not encourage anyone to be careless with the use of any non-food-tested dye, including natural dyes. However, there's no reason to be more concerned about Procion MX dyes than about other household chemicals such as dishwasher detergent or chlorine bleach, both of which are more hazardous. They are safe to use with the normal precautions of gloves, etc. I would be much more concerned about job exposure in a manufacturing industry, such as the manufacture of rubber, or about exposure to cigarette smoke.
In her book, Monona Rossol has little concern about dangers of fiber reactive dyes, aside from their allergenicity to people who breathe too much of the dye powders, but she warns that some anthraquinone -based dyes, which are found in many different classes of dyes, might be found to cause cancer. The anthraquinone-based dyes do not contain an azo linkage, so they are not azo dyes. Among the Procion MX dyes, Procion Blue MX-R, which is Colour Index Reactive Blue 4, is an anthraquinone-based dye; it is duller in color than the triphenodioxazine dye Procion Blue MX-G, which is Colour Index Reactive Blue 163. Interestingly, some natural anthraquinone pigments, such as those found in the rhubarb plant, have cancer-preventative properties, so it's not obvious that all such dyes will be a concern. Reactive blue 4 has not been found to be a carcinogen; we don't know whether it might be if large-scale feeding tests were performed in animals, so it is, as always, best to avoid excessive careless exposure to the dye.
For further safety information on this site, see:
• "Specific dyes to avoid" and
• "Dye Safety FAQs"
• "Specific dyes to avoid" and
• "Dye Safety FAQs"
For more information about the specific dyes you use, be sure to request the MSDS for each color you order from your dye supplier, or use my page of "Which Procion MX colors are pure, and which mixtures?" to learn the generic Colour Index names for the unmixed dye colors, so that you can search for MSDS or other safety information elsewhere. You can also see MSDS pages that have been supplied by Dharma Trading Company (look for "Dharma Fiber Reactive Procion Dyes"), or the MSDS pages supplied by PRO Chemical & Dye (look for "MX Fiber Reactive Dyes"), or, for Jacquard Products' Procion MX dyes, at Blick Art Materials (the MSDS links appear to the right, next to each color name). For the proper use of any dye, PRO Chemical & Dye's Studio Safety Guidelines are a good resource.
Best wishes for your cancer treatment. I see no reason why you should not continue to enjoy using Procion dyes, while avoiding the use of any chemicals that may increase your chances of developing another cancer.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Friday, June 10, 2011
I'm having trouble with dyeing plush. What can you recommend to dye this kind of fabric?
Name: Mayra
Country or region: Brazil - SP
Message: Hello, I am a fursuiter here in Brazil and I'm having trouble with dyeing plush. What can you recommend to dye this kind of fabric?
Currently I use airbrush and acrylic ink, but it comes off, with time.
What is the fiber content of the plush? This is the real key to the problem. If you can find a real dye that will work on your fiber content, then the results will be better and longer-lasting than acrylic-based inks, but you must find the right type of dye for the fiber, and some dyes can be difficult to obtain locally in the small quantities that you need.
Cotton velour is particularly easy to dye, as are silk/rayon velvets. The best dye for cotton is a fiber reactive dye, such as Procion, Remazol, or Drimarene dye, but even an all-purpose dye will work, if you don't launder the dyed fabric too many times, and, when you do launder it, use only cool water, by hand.
If you can find some nylon fleece, if it is not coated with a surface finish, then it will be very easy for you to dye. The best kind of dye for nylon is called acid dye, because it is used by heating the fabric and dye in water to which a mild acid, such as distilled white vinegar, has been added. Although it is a synthetic fiber, nylon is dyed using the same dyes and methods as wool. Since all-purpose dye contains an acid dye, it, too, works pretty well on nylon, though it's not the best. Antron nylon fleece has been very popular for use in making puppets, and it dyes very well, if you get the right kind of dye and use the correct recipe, but apparently its manufacture has been discontinued.
Unfortunately, synthetic furs are made from fibers that are much more difficult to dye than natural-fiber velvets and velours. The dyes that work well on cotton, silk, and wool all fail completely when applied to polyester or acrylic. The best dye for synthetic furs is a special kind of dye called disperse dye, which was developed for use on synthetic fibers. It works for polyester, nylon, acetate, acrylic, and modacrylic. It cannot be applied in cool water; polyester, acrylic, and modacrylic must be heated to boiling (100°C), with the dye, for an extended time, at least half an hour, while nylon and acetate should be heated to 85°C, or at least 50°C for paler shades. (See, for example, the forum post, "tests with iDye Poly".) Before you even try to buy this dye, test a small swatch of your plush fabric to see whether it can tolerate being boiled. Its texture may change significantly.
The most difficult thing for you about disperse dye will be finding a source to buy it. If you do a web search for disperse dye, narrowed by your location, you may find contacts for buying large industrial quantities of disperse dye. The quantities involved are far larger than would be practical. While some dye manufacturers will sell buckets containing as little as one pound of dye powder for each color, it's more common to see a minimum order of 5 kilograms. Try contacting Dystar Brazil, which has an office in São Paulo; their minimum order size is probably an immense 5 kilograms per color, but perhaps they can refer you to a company to whom they sell dye, who might be able to sell smaller quantities to you.
Small crafts and art supply shops may sell dyes in small quantities, but it is rare to find any that carry disperse dye. Look for Jacquard iDye Poly; if you find a local shop that sells any dye or paint made by Jacquard Products, ask them if they can order iDye Poly for you. (Don't let them order plain iDye, without the Poly in the name, because, while iDye Poly is disperse dye for synthetic fibers, plain iDye is a direct dye for natural fibers only.)
Another form of disperse dye that may be easier to find is a disperse dye crayon. These are sold in crafts stores or sewing supply stores as "Fabric transfer crayons". They are used by coloring heavily onto paper, then ironing the design onto your fabric. The results of ironing onto fur are difficult to predict, but it may be worth a try.
If you cannot find disperse dye anywhere in your area, and if you cannot find a mail-order source within your country, then you will have to consider buying it internationally. Shipping costs can be very high, but sometimes, if you use the telephone to order, you can request a slower economy form of shipping that will costs much less. I do not know what limitations Brazil may place on the importation of art supplies, nor what customs fees they may charge. I would recommend that you order disperse dye from PRO Chemical & Dye or Aljo Mfg in the US, both of which have much lower prices for dyes than most small-quantity dye retailers, or perhaps Kraftkolour in Australia. (See my page, "Sources for Dyeing Supplies Around the World", for contact information for these and other suppliers.)
If you can't obtain disperse dye, or if your fabric will not tolerate boiling water, then your best solution may be the airbrushed acrylic paint that you are already using, in spite of your problems with its wearing off. Note that some acrylic paints should not be diluted much; if they are diluted with too much water, then they will not bind well to the underlying material, and will wear off much more quickly. Jacquard Products fabric paints, for example, which are based on an acrylic binder, must be diluted with no more than 25% water, by volume, for airbrushing. It's possible that you could get longer-lasting results from a different brand of acrylic paint than the one that you are currently using, or by diluting it less, if doing so does not make it too thick to use in the airbrush.
(Please help support this web site. Thank you.)
Thursday, June 09, 2011
Do you still have Dylon Run Away?
I don't believe that Dylon Run Away is available in the US now, but a nearly identical product, Rit Color Remover, is easy to find throughout the US. Look at the grocery store or pharmacy; if you can't find it there, a sewing store such as Joann's should have it.
Dylon Run Away is based on sodium hydrosulfite plus sodium carbonate (washing soda). Other brands with similar formulations include Rit Color Remover, Tintex Color Remover, Dylon Run away for Whites, and Carbona Color Run Remover.
Each of these is safer for clothing than chlorine bleach, which contains sodium hypochlorite. Hypochlorite will permanently damage synthetic fibers such as nylon, spandex, and polyester, as well as natural animal-based fibers including wool and silk. The only fibers for which I recommend chlorine bleach are 100% plant-based fibers, such as 100% cotton, linen, or hemp.
There is one exception to my preference for these color remover products over chlorine bleach. The indigo dye that is used to dye denim for blue jeans does not fade when you use Dylon Run Away, Rit Color Remover, or any of the other similar color removers. The only dye remover that works on blue denim is chlorine bleach.
For more information, see "What chemicals can be used to remove dye?".
(Please help support this web site. Thank you.)
Wednesday, June 08, 2011
Would it be best to try to lighten the color of the cotton before trying to dye it, or to simply dye over the pink?
Name: Katie
Country or region: USA
Message: I have a fairly bright, bubblegum-y pink cotton dress with white detailing and a polyester lining. I'd like to dye the dress purple. I understand that the polyester lining likely won't take color, the detailing will probably turn out a darker color, and that I should use a cold water fiber reactive dye for best results. Would it be best to try to lighten the color of the cotton before trying to dye it, or to simply dye over the pink?
My apologies if this has been asked already. I looked through previously asked questions and didn't find anything that I felt really answered my question. Any input you have will be greatly appreciated. Thank you in advance!
If it's an orangish, peachy pink, then I think you should lighten it first, using Rit Color Remover or Jacquard Color Remover or some such product. (See "What chemicals can be used to remove dye?".) This is because yellow and orange, when mixed into purple, tend to turn it brownish, since yellow is the complement color (opposite on the color wheel) of purple. I would avoid the use of chlorine bleach, because its hypochlorite will tend to stain the polyester and unattractive dull yellowish color that cannot be removed.
However, if the dress is a clear pink with no orangishness to it at all, and if it's lighter than the purple you want, not darker than it, then there should be no need to remove any of it first before trying to dye it purple. If you overdye a bright deep pink with blue, then it will make purple. If the pink is less intense, it will have less effect, and you should just use a purple dye.
Chances are good, by the way, that the detailing may be made from polyester or nylon, neither of which will take the fiber reactive dye under the same conditions that cotton will. So, the detailing will probably end up white, not darker, depending on what it's made of. If it's made of rayon, it will probably end up darker, and it will be a bluer purple than the rest of the dress, without the original pink color to cover.
You could dye the whole dress at the same time, using a large cooking pot, iDye for the cotton, and iDye Poly for the polyester and nylon. You could correct the poor washfastness of the direct dye in iDye on the cotton with a commercial dye fixative such as Retayne or iDye Fixative. However, the boiling water would have a very strong tendency to shrink the cotton fabric. When a lined dress shrinks, the lining never shrinks at the same rate as the outer layers, so the shape of the dress is ruined. I think your choice, using a cool water fiber reactive dye, is best, and it's certainly a lot less trouble, and less expensive, too, since it won't require you to invest in a dyeing pot.
The easiest way to dye the dress a solid color will be in a top-loading washing machine. See "How can I dye clothing or fabric in the washing machine?".
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Tuesday, June 07, 2011
Will a different dye work to color both the clothing and its stitching?
Name: Mag
Country or region: UK
Message: Hello,
I dyed a top using Rit all-purpose dye and all went well apart from stitches which darkened a little but stayed the same colour. Now I have almost identical top (the same brand and line so the stitching will be the same) and would like to know whether it is possible to solve this problem by getting a different type of dye?
This is a problem we see frequently. Almost all clothing, these days, is constructed with seams sewn with polyester thread. Polyester will not take any sort of dye that works on natural fibers; not only does it require a special sort of dye, known as disperse dye, it also requires high heat, often half an hour or more of being boiled in the special polyester dye.
Rit all-purpose dye contains direct dye, which works on cotton and silk, plus leveling acid dye, which works on wool, silk, and nylon. It does not contain any disperse dye, so it does not dye polyester, except sometimes for a light temporary stain.
If your top does not contain any spandex (also known as elastane), which must be protected from heat, then you might want to consider a combination of dyes, packaged separately by Jacquard Products, called iDye and iDye Poly. iDye Poly is a disperse dye, which works only on polyester and other synthetic fibers. iDye (without "Poly" in the name) is a direct dye, similar to the direct dye in Rit, so it works only on natural fibers and on viscose rayon. Direct dye is not nearly as wash-resistant as the fiber reactive dye I prefer, but that problem can be fixed by applying a commercial dye fixative such as iDye Fixative or Retayne.
In the UK, a good online source for Jacquard Products is George Weil. They carry all eight colors of iDye Poly, which can be mixed with iDye for natural fibers and applied in a pot of boiling water, in a single step. I don't see regular iDye on George Weil's website, but they should be able to order it if requested, since they carry other Jacquard products, or you could substitute the Deka L direct dye that they sell. The iDye or Deka L dye will color only the natural fibers in your top, while the iDye Poly colors the polyester threads.
After you have completed your dyeing, you can apply Fixitol P, which is the brand of cationic dye fixative that George Weil carries. Without Retayne, Fixitol P, or another brand of cationic fixative, both Rit dye and iDye can fade quickly in the laundry. (This is one of the reasons why I prefer fiber reactive dye to direct dye.)
If your top's fiber content includes elastane or spandex, then you will not be able to use iDye Poly, since it must be applied in boiling water, while elastane is easily damaged by hot water. In that case, you will have to either accept the contrasting color of the undyed thread, or obtain clothing that has been sewn with cotton thread. The best way to do this may be to find a local seamstress who is willing to sew clothing for you, using only dyeable materials. Commercial clothing sewn with cotton thread may be labeled PFP (Prepared For Printing), RTD (Ready To Dye), or PFP (Prepared For Printing). Unfortunately, I don't know of good sources in the UK for clothing blanks that are suitable for dyeing, though it seems as though they should exist. In the US, the easiest place to find clothing blanks that have been sewn with cotton thread is Dharma Trading Company.
(Please help support this web site. Thank you.)
Monday, June 06, 2011
Does batik fabric from India need to be washed before I use it in a quilt top?
Name: Loretta
Country or region: US
Message: Does batik fabric from India need to be washed before I use it in a quilt top?
I wouldn't use any fabric in a quilt top without washing it first, unless the design is such that it won't matter at all if the colors run from one piece of fabric to another. At this point, you have no idea whether the dye in this fabric will run badly or not. A real batik will certainly have less of a tendency to run than others, if the boiling method was used to remove the batik wax and if fiber reactive dyes were used, but you might have a printed faux batik, instead.
In fact, if you're piecing any fabric into a complex quilt top, instead of just using the whole fabric for the quilt top, I wouldn't use it without testing it. To test dyed fabric for washfastness, dampen it, place it between two white cloths, and then iron it dry with a hot iron. If color transfers to the white fabric during this test, you know there's a problem that you'll need to fix, either by repeated washing in hot water, or by using a commercial cationic dye fixative, such as Retayne, or both. (See my page on "Commercial Dye Fixatives".)
Even for an unpieced quilt with a backing of the same color, you should pre-wash the fabric, I think, if only to pre-shrink it. I imagine that a whole cloth quilt, made without piecing at all, would get weirdly puckered later, if it shrinks after quilting. I would wash the fabric in hot water to be sure that it is pre-shrunk.
Not all dyes respond to Retayne and other commercial dye fixatives. The fixatives do not work on a particular type of dye known as vat dyes, including indigo. Most non-washfast dyed fabric from India seems to be made with another type of dye, known as direct dye, however. Direct dye responds well to Retayne, becoming considerably more washfast after treatment.
Sometimes you hear that the dyer's detergent Synthrapol will set the dye in fabric. Unfortunately, this is not true. Synthrapol is an excellent detergent, ideal for removing excess dye since it is pH-neutral and free of optical brighteners and fragrance, but it has no magical dye-setting properties. Using very hot water, even without a detergent, is more effective for dye removal than using Synthrapol in cooler water. (See my page, "What is Synthrapol?".)
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Sunday, June 05, 2011
Name: Sue
Country or region: Long Island, NY, USA
Message: I bleached some old lace (maybe 50 year old lace) and then I read that you never do this - the bleach eventually rots the lace. I am uncertain about the amounts of things. And, is it too late to do anything about the bleach now? Thank you.
I think it would be a good idea to soak the lace in a chemical that will neutralize any remaining hypochlorite from the bleach. The most convenient one to use is hydrogen peroxide, the 3% dilution that is sold at the pharmacy for use as an antiseptic. Soak the lace in the peroxide (straight or diluted with a quart or two of water) for ten minutes or so, then rinse and wash as usual.
If you happen to have Anti-Chlor (sodium metabisulfite, used in brewing wine and beer at home) or Bleach Stop (sodium thiosulfate), those work very well for this purpose, too. Peroxide is just as good and is easier to obtain locally in a hurry. I do not recommend the use of an acid, such as vinegar.
For more information, please see my page, "How can I neutralize the damaging effects of chlorine bleach?".
(Please help support this web site. Thank you.)
Friday, June 03, 2011
Can you suggest a monofunctinal dye that would give a darker shade than Reactive Blue 19? Name: Marlin
—ADVERTISEMENTS—
Victor B. Ivanov's

Reactive Dyes in Biology and Medicine
Explains use of reactive dyes for staining proteins or carbohydrates
Waring and Hallas's

The Chemistry and Application of Dyes (Topics in Applied Chemistry)
includes recipes for synthesizing reactive dyes
Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
John Shore's

Cellulosies Dyeing
Useful information about the chemistry of reactive dyes, and other dye types
Message: Thank you for getting back to me. [link] You seem to know more about reactive dyes than anyone I've come across. I have used Reactive Blue 19 (monofunctional vinyl sulfone) with success to dye protein molecules in solution. As the protein concentrations are not high, I was hoping to find a darker dye to mark them. My natural thought was to try a black dye, presumably the darkest color available. Bifunctional dyes cause cross-linking between protein molecules in solution, rendering them insoluble. Can you suggest a monofunctional dye that would give a darker shade than Reactive Blue 19?
That's an interesting question. Perhaps surprisingly, black dyes are not always much darker in color than dyes of other hues. It is necessary to use a much larger quantity of black dye powder, when dyeing textiles, as you would use for any other color, because otherwise black dyes tend to produce undesirably light hues, shades of gray rather than black, usually tending toward a non-neutral color such as blue, purple, or green.
What you want is not a dye that is darker in color, but one which has a higher tinctorial strength. I think the best results I've had along these lines, in dyeing fabric, have been with navy blue dyes. Among the dichlorotriazine dyes, I've obtained somewhat darker results, with less dye, using Reactive Blue 9, which I purchased from Standard Dye, or reactive Blue 109, which is sold as Procion Cobalt Blue by Dharma Trading Company, and as Pro Mixing Blue by PRO Chemical & Dye. (If you order from Standard Dye, make it clear to them that you want only the Colour Index number you agree to, not a mixture which produces approximately the same hue.)
The monofunctional Cibacron F dyes (now renamed to Novacron by their new manufacturer, Huntsman Textile Effects) have been claimed to have higher tinctorial strength than the older Procion dyes, based on the selection of the chromophores. If you look at Sacracron F dyes at PRO Chemical & Dye, which are the Cibacron/Novacron F type, the color Deep Navy (Reactive Blue 184) is obviously much more intense, when used to dye a sample at a 4.0% depth of shade, than the color National Blue (Reactive Blue 182) at the same strength. The Rich Black in the same line of colors required two and a half times as much dye powder to get the intense black shown in its color chip on the same page. Reactive Blue 184 might be a good choice for you to test first. ProChem is a handy place to order the dye from, since they will sell you a jar as small as two ounces.
Inconveniently for your purposes, newer bifunctional dyes are always claimed to have greater tinctorial strength than monofunctional dyes, perhaps because of reduced loss of reactivity due to degradation of the reactive section of the dye molecules after manufacture.
Many dyers have observed that the best acid dyes produce more intense colors on protein fibers than the best reactive dyes. I wonder if that would be another answer for you. Acid dyes are used to dye protein fibers, never cellulose fibers, unlike reactive dyes, which are used to dye both. The bonds that are formed between acid dye and fiber are weaker than the covalent bonds formed between reactive dye and fiber, which might be a problem for you. (See "What kinds of chemical bonds attach dyes to fibers?".)
I don't know whether acid dyes are typically used in quite the same way as you are using your reactive dyes, but of course there are many acid dyes frequently used as protein stains. For example, Coomassie Brilliant Blue, which is also known as Colour Index Acid Blue 90, is commonly used for quantifying protein. I used it that way myself as an undergraduate. It's one of the acid dyes commonly used by hand dyers, sold by PRO Chemical & Dye under their name Wash Fast Acid Brilliant Blue 490.
The explanation for why acid dyes should produce more intense hues than reactive dyes goes as follows. When a reactive dye bonds to cellulose, it can attach to any one of the many glucose molecules in the cellulose chain, because it reacts with an OH group. The large number of dye sites make for a high maximum intensity of color. In contrast, when a reactive dye bonds to protein, forming the covalent bond of a reactive dye at a high pH, the only amino acids available for the reaction are those which contain an OH in their side chain, such as serine, in addition to the amine group or carboxylic acid group at the end of the final amino acids, at either end of the protein molecule. Since only a minority of the amino acids in, for example, a silk molecule, can provide an OH group, a reactive dye may produce a somewhat weaker color on protein than on cellulose. (See the Dye Forum discussion, "Silk Dyeing Question.) In contrast, an acid dye forms hydrogen bonds, instead of the covalent bond of a reactive dye, and can attach along the chain of the protein molecule wherever hydrogen bonding is possible.
To complicate matters considerably, the reactive dyes we use on cellulose, when applied to protein fibers at a low pH, do not form covalent bonds as reactive dyes, but instead form hydrogen bonds and salt linkages like an acid dye. A reactive dye can easily be forced to act as an acid dye on protein fibers, if the conditions are appropriate, since the chromophore regions of reactive dyes, without the reactive region, has the same sort of structure as any acid dye. When it bonds as an acid dye, a reactive dye is less firmly attached to the protein molecule than when it bonds as a reactive dye. At what pH do you attach your Reactive Blue 19 to your protein? Can you tell me what your general procedure is for using Reactive Blue 19 as a stain for proteins in solution?
Many dyers feel that the best of the dyes commonly used for hand-dyeing protein fibers are the ones in the Lanaset series. They are known for producing more intense colors, with a smaller amount of dye, than any of the other dyes that are readily available to hand dyers. They are more expensive than many other dyes for protein fibers, but their high tinctoral strength, and other properties including much higher washfastness than other acid dyes, make them worth the cost. I wonder if this would make them more suitable for your purpose. Take a look at my page, "Which Lanaset dye colors are pure, rather than mixtures?. Lanaset dyes are said to contain both acid dyes and reactive dyes, but I believe that the reactive dyes that may be sold along with Lanaset dyes are all actually Lanasol dyes. Both types of dye in the Lanaset series are considerably more wash-resistant than other dyes used for protein-containing textile fibers. While most acid dyes are tested for washfastness on protein-based fibers by washing the dyed material in lukewarm water, at 40°C, Lanaset dyes resist being washed off of fibers when washed in hot water, at 60°C. Unlike other reactive dyes, the Lanaset dyes are applied at a mildly acid pH.
A common observation for dyeing with reactive dyes, such as vinyl sulfone or Procion dyes, is that the dyebath remains dark in color after the dyes are fixed on the fiber; extensive washing is required to separate unattached dye molecules from the dyed substrate. In contrast, acid dyes typically "exhaust" onto the fiber, forming a close association with the molecules of the even before they have fully bonded to it, leaving a colorless dyebath behind. I would imagine that this different behavior would be interesting when staining proteins in solution.
Lanasol dyes, formerly made by Ciba Geigy, now (like the Cibacron/Sabracron/Novacron dyes) made by Huntsman Textile Effects, are reactive dyes for wool that are based on a alpha-bromoacrylamido group, which reacts with the protein in wool by a nucleophilic addition reaction. Unfortunately for your purposes, I've just read in Robert M Christie's book, Colour Chemistry, that there is a possibility that the Lanasol dyes might be considered as bifunctional, if the second bromine atom in the dye's reactive group also reacts with the protein, via nucleophilic substitution, which would in principle, he says, lead to cross-linking of the fiber.
My conclusion is that you should consider testing Reactive Blue 184 from PRO Chemical & Dye as your next reactive dye, and consider whether one of the Lanaset acid dyes might work for you.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Thursday, June 02, 2011
Why do some dye color mixtures bleed a different color around the edges?
Name: Nate
—ADVERTISEMENTS—
150 different colors from one kit!
Tulip Custom Dye Color Kit








Synthetic Dyes for
Natural Fibers
By Linda Knutson


Tulip Custom Dye Color Kit


Synthetic Dyes for
Natural Fibers
By Linda Knutson
Country or region: USA
Message: Hi, I have been dyeing for several years now and have been working on mixing my own colors for a little over a year now. I am trying to figure out why some colors I mix will bleed or leach another color—like my browns always have green or yellow around them, and some reds have yellow run from them, for a few examples. Have you ever heard anything like this? Could I be doing something wrong? Any suggestions you have would be greatly appreciated.
Yes, I know exactly what you're talking about. It's caused by the fact that the different dye colors are each produced by a different chemical, with different properties. To avoid this separation of colors, you can choose different colors to use as mixing primaries.
Some reactive dyes react almost instantly with the fiber you're dyeing, almost as soon as they hit it, assuming that the pH is high enough (thanks to the soda ash presoak). This means that they do not have time to creep around on the fabric. As soon as they come into contact with the fiber, they become permanently attached to it. In contrast, some other reactive dyes are much slower to form a chemical bond to the fiber. This means that they have plenty of time to spread out, and end up moving farther on the fabric.
If you make a purple by mixing fast-reacting Red MX-8B, which is commonly known as Fuchsia, with slow-to-react Turquoise MX-G, then the fuchsia molecules will start bonding to the first fibers they come into contact with. Fewer of the turquoise molecules will form bonds in the first few minutes; since they don't bond immediately, there's plenty of time for them to soak along the fiber. The result? A blue halo around your purples. Sometimes that's a very nice effect, but other times you just want a solid purple, without any bleeding of the blue around it.
The color separation happens only in direct application of the dye, such as tie-dyeing or dye painting; if you do immersion dyeing with a high water ratio, all of the parts of the fiber get exposed to all of the dye colors, so you get only a single solid color.
Fortunately, you have more than one choice of a red Procion MX type dye, and more than one choice of blue. If you want less in the way of blue halos around your purple, then use Red MX-5B (called light red, magenta, or mixing red) instead of Red MX-8B (fuchsia). Red MX-5B happens to react more slowly than Red MX-8B, so you will experience less trouble with halos. Another alternative is to use an unmixed single-hue purple dye, the lovely Violet MX-2R, which is sold as Grape from many dye suppliers.
If you mix a bright fire engine red by mixing Red MX-8B with Yellow MX-8G, you will end up with yellow halos around the red sections of your tie-dyes. This can be a very nice effect, but it won't always work for you. For a red that doesn't separate, it's better to mix Red MX-5B with Orange MX-2R.
Your browns that end up with yellow or green around them are probably based on fuchsia. Let me suggest that you obtain some orange dye, and make browns by adding a navy blue dye to it. The best orange for mixing dark dull colors, such as dark brown and black, is the terracotta orange whose generic MX code is brown MX-GRN. As it happens, Dharma Trading Company does not sell this dye, but lots of other dye suppliers do. PRO Chemical & Dye sell it as 515 Burnt Orange, while Jacquard Products sells it as 016 Rust Orange. There are several good navy blue dyes for mixing dark colors. Blue MX-2G is a good one, sold just about everywhere that you can buy Procion dyes; Dharma and Jacquard sell it as Cobalt Blue, while ProChem sells it as Mixing Blue. For different shades of brown, try the medium blue colored Blue MX-G, known as Cerulean Blue at Dharma and Jacquard, and as Intense Blue at ProChem, or the bright orange Orange MX-2R, which is called Deep Orange, Strong Orange, or Brilliant Orange, depending on the supplier.
Many of the Procion MX dyes that you can buy are mixtures, made of two or more different colors of dyes already. There are only about a dozen pure unmixed single-hue Procion MX dyes readily available. To see which ones these are, and what their names and catalog numbers are at several of the major dye suppliers, see my page, "Which Procion MX colors are pure, and which mixtures?".
Several years ago, I posted some data about how quickly some of the Procion MX dyes react, whether with a textile fiber or with water, in the Dye Forum; see "reactivity of Procion MX type dyes". Unfortunately, some of our favorite dyes are missing from the list. If there's a particular dye whose performance you'd like to know more about, posting on the Dye Forum is an excellent way to get different dyers' experiences.
You will also see less color separation if you use a thickener in your dyes, such as sodium alginate; see "Sodium alginate, Superclear, and other dye thickeners". Some tie-dyers always use sodium alginate, while others don't like to use thickeners at all. Give it a try sometime and see how you like it.
As a curiosity, you will see entirely different results with the same dyes when you fix the dyes only after they had a chance to soak into the fabric for a while, because then the dyes that travel farther will be the smaller and lighter dye molecules, rather than the ones that are the fastest to react. Instead of pre-soaking in soda ash, you can apply dye to shirts that are either dry, or dampened only with water, let the dye dry completely (don't use urea in mixing the dyes, for this method), then paint on the sodium silicate solution that is called either Water Glass or After Fix. It's an impractical method for fixing multiple tie-dyes, but sometimes a good choice for dye painting. Similarly, most of us prefer to add soda ash after the dye when doing low water immersion dyeing, though some do it the other way around. See "How to Do Low Water Immersion Dyeing".
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)
Wednesday, June 01, 2011
Can you suggest a monofunctional reactive black dye? Name: Marlin
—ADVERTISEMENTS—
Victor B. Ivanov's

Reactive Dyes in Biology and Medicine
Explains use of reactive dyes for staining proteins or carbohydrates
Waring and Hallas's

The Chemistry and Application of Dyes (Topics in Applied Chemistry)
includes recipes for synthesizing reactive dyes
Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
John Shore's

Cellulosies Dyeing
Useful information about the chemistry of reactive dyes, and other dye types
Message: I am looking for a monofunctional reactive black dye, preferably a vinyl sulfone. I have looked at Reactive Black 5, but it is a bifunctional vinyl sulfone dye (it has two vinyl sulfone reactive groups on the molecule). A bifunctional dye will not work in my application. Can you suggest a monofunctional reactive black dye?
Most lines of reactive dyes lack a good dark black altogether; reactive black dyes available for sale are usually mixtures of several different colors. Reactive black 5 is the only unmixed black reactive dye I've ever worked with.
On my page about vinyl sulfone dyes (a type of reactive dye), there are only two unmixed black dyes listed, Reactive Black 5 and the less common Reactive Black 31. Reactive Black 31 can be purchased for use as a textile dye from Granat Farvekompagniet, in Denmark. Before you order it, note that I suspect that it, too, is a bifunctional dye.
Is there any particular reason why another color, such as navy blue, will not do? There are a great many more reactive navy blue dyes. There is even a little bit of overlap in names or colors between some of the reactive blacks and some of the reactive dark blues. In fact, reactive black 5 itself has a distinctly bluish cast.
Here's a list of the unmixed reactive black dyes for which I have some information, in order of their Colour Index names:
1. reactive black 1, a monochlorotriazine dye (Procion Black H-G). Appears to be a chromium-cobalt complex.
2. reactive black 5, a vinyl sulfone (Remazol Black B). Bifunctional.
3. reactive black 8, a monochlorotriazine (Procion H-N). Appears to be a chromium-cobalt complex.
4. reactive black 13 (Cibacron Grey G-E).
5. reactive black 23 (Cibacron Pront Grey 2 B).
6. reactive black 26, a methylsulfonyl dye (Levafix Black P-G).
7. reactive black 31, a vinyl sulfone (Remazol Black RL). Looks bifunctional.
8. reactive black 39, a monochlorotriazine (Procion H Blue P2R). Looks monofunctional.
9. reactive black 49. (Dycrofix Blue P3R). Looks monofunctional. Actually reactive blue 49.
10. reactive black 160, monochlorotriazine (Black HEBL). Looks bifunctional. Actually reactive blue 160.
There is no reason to believe that my list is complete, though these are the most likely to be locatable.
More details on the above list:
1. Reactive Black 1, Procion Black H-G. CAS Number: 12236-77-0. Molecular Formula: C23H13ClN8Na2O10S2 [this formula omits the chromium and cobalt]. Synonyms:
1-Naphthalenesulfonicacid,4-[[6-[(4-amino-6-chloro-1,3,5-triazin-2-yl)amino]-1-hydroxy-3-sulfo-2-naphthalenyl]azo]-3-hydroxy-7-nitro-, chromium-cobalt complex (9CI); C.I. 17916; Cibacron Black BG; Cibacron Black BG-A; Cibacron Black FBG-A; Cibracron Black BG-A; Procion Black H-G. Available from Sigma Aldritch as Cibacron Black FBG-A.
2. Reactive Black 5, which you've already rejected for its bifunctionality, is the easiest-to-find reactive black dye (that is not a mixture of other colors). It is symmetrical with its two reactive ends, as shown in the molecular structure drawing below.

Its full chemical name is [2,7-naphthalenedisulfonic acid, 4-amino-5- hydroxy-3,6-bis((4-((2-(sulfooxy)ethyl)sulfonyl)phenyl)azo)-tetrasodium salt], while its formula is C26H21N5Na4O19S6, CAS Reg. No. 17095-24-8. The formula will be useful below for comparing to a dye for which little other information is available.
3. Reactive Black 8, also known as Black DN or Black HN, is a monofunctional black reactive dye, in the monochlorotriazine (Procion H) group, but, like Reactive Black 1, it is described as occurring in a 1:2 chromium-cobalt complex of two dye molecules per metal atom. When complexed to chromium alone, the dye produces blue, while when complexed to cobalt alone, it is reddish. Synonyms: C.I. 18207; Cobalt 5-[(4-amino-6-chloro-1,3,5-triazin-2-yl)amino]-4-hydroxy-3-[(2-hydroxy-5-nitrophenyl)azo]-2,7-naphthalenedisulfonic acid complex; Basilen Black P-BR; Cibacron Black B-D; Drimarene Black P-BL; Helaktyn Black DN; Kayacion Black P-N; Ostazin Black H-N; Procion Black H-N; Procion Black P-N; Reactive Black K-BR; Reactive Black MN. CAS Registry Number 12225-26-2.

[picture from Industrial dyes: chemistry, properties, applications, by Klaus Hunger, p. 312 (John Wiley and Sons, 2003).]
I have another picture of Reactive Black 8; unfortunately I cannot determine its source. What's interesting about it is that it contains essentially the same dye molecule as the picture above, but with no mention of the chromium or cobalt, nor of a complex of two or more dye molecules:

The fact that a dye takes the form of a dimer complexed with chromium and/or cobalt makes little difference to most purposes, but might be a real problem in cases in which only a monofunctional dye will do. Having seen these two different drawings for what is essentially the same dye, I think we may need to wonder about whether any single monofunctional dye is involved in a similar complex.
4. Reactive Black 13. Cibacron Grey G-E; Hicion Grey HE-G; CAS 12225-31-9. I have no more information on this dye. Sigma Aldritch carries it; contact them for more information.
5. Reactive Black 23. Cibacron Pront Grey 2 B. I have no more information on this dye. Sigma Aldritch carries it; contact them for more information.
6. Reactive Black 26. Levafix Black P-G; Levafix Black E-2G. CAS 12731-61-2. I have no more information on this dye.
7. Reactive Black 31 is listed as being in the Remazol series. Synonym: Black RL. CAS# 12731-63-4
I do not have the molecular structure of the dye reactive black 31, but I have a molecular formula:
C29H20N6O17S4•4Na
Comparing that to the molecular formula of reactive black 5, C26H21N5Na4O19S6, the two look similar in size, which leads me to suspect that reactive black 31 may also be a bifunctional dye.
It is carried by Jagson and Shivitex; the latter provides fastness ratings for this dye.
8. Reactive black 39 is in the Procion H series, sold by Jagson as Dycrofix Blue P2R (their color chip shows a pale gray). Synonyms: Jagson's Dycrofix Blue P2R; Ostazin Blue H-2G; possibly Cibacron Navy G.

Picture source: Environmental chemistry of dyes and pigments, Abraham Reife, H. S. Freeman, page 12 (Wiley-Interscience, 1996).
9. Reactive black 49. This appears to be a misprint for Reactive Blue 49. Jagson shows Reactive Blue 49 with a black color chip, so it's worth considering in spite of its name.
Synonyms: 2-Anthracenesulfonicacid,1-amino-4-[[3-[[4-chloro-6-[(3-sulfophenyl)amino]-1,3,5-triazin-2-yl]amino]-2,4,6-trimethyl-5-sulfophenyl]amino]-9,10-dihydro-9,10-dioxo-,sodium salt (1:3); Cibacron Blue 3R; Cibacron Blue P 3R; Cibacron Brilliant Blue 3R-P; Helaktyn Blue D 3R; Kayacion Blue P 3R; Levafix Blue PN 3R; ProcionBlue P 3R; Procion Brilliant Blue H 3R;. CAS 72927-99-2. Molecular Formula: C32H26 Cl N7 O11 S3 • 3 Na

Structure from http://www.lookchem.com/cas-729/72927-99-2.html. It looks like a Procion H type (monochlorotriazine).
10. Reactive black 160 is sold by Jagson as Black HEBL, with a medium gray color chip. (See http://www.jagson.com/reactive_dyes.htm). They don't give any other identifying numbers. Chemicalregister.com lists a reactive black 160, but it appears to be a misprint for reactive blue 160. Here's their image for reactive black 160:

Here's an image of reactive blue 160, from guidechem.com, which is easier to read but does appear to be the same molecule: you can see two aminochlorotrizine (monochlorotriazine) groups in it.

I'm guessing that Reactive Black 1 and 8, in their chromium/cobalt complexes, will be as unsuitable for your purposes as the bifunctional dyes. Perhaps one of the two Cibacron Grey dyes from Sigma Aldritch, Reactive Black 13 or 23, will meet your needs; otherwise, your best bets from this list would appear to be either Reactive Black 39 or Reactive Blue 49.
You should be able to get more information if you contact the dye manufacturers directly; the trouble for some is finding a supplier that carries the dye.
(Please help support this web site. Thank you.)
(Please help support this web site. Thank you.)


