« 2007 December | Main | 2007 October »
Friday, November 30, 2007
any resource ideas for plain white canvas Keds sneakers (blanks for painting, so hopefully cheaper than keds)
Thursday, November 29, 2007
I have two all-white Chicago Cubs baseball caps that I'd like to dye a navy blue
Wednesday, November 28, 2007
planning to dye a sweatshirt that has a silkscreened design. will the dyeing process cause any shrinkage in this kind of garment?
Tuesday, November 27, 2007
How can I dye blue overalls to make them coral?
Monday, November 26, 2007
will the cold prevent the dyes working?
Sunday, November 25, 2007
My tub is now a nice shade of blue and scrubbing it with Ajax is NOT helping. Any ideas on how I can get it off
Saturday, November 24, 2007
I received a bleach stain on my beige cotton sweater. I would like to dye the entire sweater. Can this be done?
Friday, November 23, 2007
toxicity and environmental damage associated with logwood and other natural dyes
Thursday, November 22, 2007
backstaining of dye in batik, and unwanted pale colors in batik
Wednesday, November 21, 2007
the chemical, biological and physical processes and properties that generate the colours in clothing
Tuesday, November 20, 2007
Can I save soda ash mixtures for other tie dying batches in the future? If so, how long can I save it for, and in what conditions?
Monday, November 19, 2007
I have about 10m of natural wool to dye black, I bought a dye and tried a small off cut it turned out brown. So, to dye a natural wool, cream in colour, black, where did I go wrong and, more importantly, what do you suggest?
Sunday, November 18, 2007
dyeing a fishing vest so that it will not scare the fish
Saturday, November 17, 2007
I want to make a rainbow swirl tie dye with black "stripes" streaming out from the center. How do I get the stripes?
Friday, November 16, 2007
My daughter painted a banner on a sheet with crayola washable paint. I would like to make it into a quilt backing/blanket. Is there any way to set the paint and make this blanket washable?
Thursday, November 15, 2007
Dyeing bamboo sheets with Procion Mx dyes: is bamboo fabric sturdy enough to use soda ash on?
Wednesday, November 14, 2007
I have a light blue cotton shirt that doesn't come in black that I found on clearance. Now I want to make the shirt a medium gray color, don't care how even the color is, just don't want it baby blue colored.
Tuesday, November 13, 2007
I would need some azo dye structure of scarlet red, navilene brown, brilliant violet, remazol navy blue, remazol black, would you send through the mail
a science fair project on artificial dyes versus natural dyes
Monday, November 12, 2007
I am trying to find a dye house to dye large quantities of baby onesies for wholesale. Do you have any resources...
Sunday, November 11, 2007
Do you make scrub sets? I have been going crazy trying to find someone that does sets and not just tops.
Saturday, November 10, 2007
Will I have problems if I try to mix all purpose dye with fiber reactive dye to make a specific color? I was unable to find Rit Kelly Green color, so I bought some green Dylon Permanent Fabric Dye.
Friday, November 09, 2007
types of dye to use for dyeing Cordura nylon motorcycle clothing
Thursday, November 08, 2007
how can we set the dye in the bingo daubers we used to color our nylon cords for making rosaries?
Wednesday, November 07, 2007
I used a clothes dye but I can't get the dye off my hands wondered how I would get it off help
Tuesday, November 06, 2007
I bought some fabric dye from Dharma Trading company and I lost the directions. I want to dye a pair of jeans and I am very nervous.
Monday, November 05, 2007
dyeing a vintage silk kimono a fluorescent color: how does the lightfastness of these dyes compare?
Sunday, November 04, 2007
I have a dark purple 100% Acetate dress. Can it be dyed black? If so, how?
Saturday, November 03, 2007
lots of questions before tie-dyeing: alginate, IPA, urea chemical water, how much dye, how long to microwave
Friday, November 02, 2007
boysenberry or violet MX-BR
Boysenberry is listed among PRO Chemical & Dye's Procion MX dyes as "PRO MX Boysenberry 802 Violet MX-BR".
The code "Violet MX-BR" appears to be a made-up MX code, possibly from Standard Dyes which makes a habit of using incorrect MX codes (witness Standard's renaming of reactive violet 14 from violet MX-2R, which describes its color nicely, to the totally bogus violet MX-G). ProChem told me, when I asked some time ago, that they did not know what its Colour Index number was, and that they would ask their supplier, but they never got back to me. They said that it is definitely a single-hue unmixed dye, which agrees with my observations.
(Don't confuse MX codes that have different color base names! "MX-BR" is meaningless without its base name of "magenta" or "violet" or "red". For example, red MX-G has nothing in common with turquoise MX-G, except that both are dichlorotriazine (Procion MX type) dyes. See "What do the letters and numbers in the code name for a Procion MX type dye mean? " for more information on how to use these codes.)
Scarlet MX-BRA is a rather uninteresting though undeniably useful manufacturer's mix of two dyes; I believe that it is orange MX-2R combined with red MX-5B, which is a good way to mix a halo-free true red, using Procion MX type dyes. It is completely different from and unrelated to boysenberry. Boysenberry 802 is distinctly bluer than fuchsia (red MX-8B), but much redder (or pinker) than reactive violet 14 (whose proper MX code is violet MX-2R). It is a little difficult to dissolve. Surprisingly, boysenberry mixes with tangerine yellow (yellow MX-GR) to make a very good blood red.
It is my best guess that boysenberry is reactive violet 13 (magenta MX-B), but it might be reactive violet 12 (violet MX-4R) or reactive red 74 (pink MX-B), both of which are on the worldwide market for the textile industry. I don't have any samples of either for testing as a comparison.
Did you know that Aljo Manufacturing is now selling the beautiful blue-violet, blue MX-7RX (reactive blue 161), in the US? It's been unavailable here for many years, except for a brief period when it was imported by Scarlet Zebra.
(Please help support this web site. Thank you.)
Thursday, November 01, 2007
1) What are dyes? 2) What is Types of Dyes? 3) How to Prepare Dyes?
any resource ideas for plain white canvas Keds sneakers (blanks for painting, so hopefully cheaper than keds)
Name: Sue
Message: Paula - any resource ideas for plain white canvas Keds sneakers (blanks for painting, so hopefully cheaper than keds)
I've found off-brand cotton canvas shoes at Target that dyed well when soaked in soda ash and then painted with Procion MX dyes. They were not particularly comfortable, since they had no arch support, but they were very inexpensive. Walmart or other discount stores might carry similar shoes. Amazon sells these Cherokee canvas oxfords for $12.99.
Amazon sells white canvas Converse shoes (see link) .
If these are cotton canvas (or hemp canvas), they should dye well, unless they
have had a water-resistant or stain-resistant finish applied to them. (Try
spilling a drop of water on the fabric; if it beads up, it is water-resistant
and thus no good for dyeing.) If you can find them without such surface
finishes, even synthetic-fiber canvases should work well with acrylic fabric
paints such as Lumiere or Dye-Na-Flow. For the greatest durability without
heat-setting these paints, you can add a product called Jacquard AirSet, which
catalyzes the acrylic reaction without
heat.
.
If these are cotton canvas (or hemp canvas), they should dye well, unless they
have had a water-resistant or stain-resistant finish applied to them. (Try
spilling a drop of water on the fabric; if it beads up, it is water-resistant
and thus no good for dyeing.) If you can find them without such surface
finishes, even synthetic-fiber canvases should work well with acrylic fabric
paints such as Lumiere or Dye-Na-Flow. For the greatest durability without
heat-setting these paints, you can add a product called Jacquard AirSet, which
catalyzes the acrylic reaction without
heat.

Keds sells some shoes that are labeled as being stain-resistant: avoid these because they will not dye well. The fact that some are labeled as stain-resistant makes it seem hopeful that the others that are not so labeled are free of this dye-repelling finish. Their white twill shoes seem like a good bet.
(Please help support this web site. Thank you.)
Message: Paula - any resource ideas for plain white canvas Keds sneakers (blanks for painting, so hopefully cheaper than keds)

I've found off-brand cotton canvas shoes at Target that dyed well when soaked in soda ash and then painted with Procion MX dyes. They were not particularly comfortable, since they had no arch support, but they were very inexpensive. Walmart or other discount stores might carry similar shoes. Amazon sells these Cherokee canvas oxfords for $12.99.
Amazon sells white canvas Converse shoes (see link)
 .
If these are cotton canvas (or hemp canvas), they should dye well, unless they
have had a water-resistant or stain-resistant finish applied to them. (Try
spilling a drop of water on the fabric; if it beads up, it is water-resistant
and thus no good for dyeing.) If you can find them without such surface
finishes, even synthetic-fiber canvases should work well with acrylic fabric
paints such as Lumiere or Dye-Na-Flow. For the greatest durability without
heat-setting these paints, you can add a product called Jacquard AirSet, which
catalyzes the acrylic reaction without
heat.
.
If these are cotton canvas (or hemp canvas), they should dye well, unless they
have had a water-resistant or stain-resistant finish applied to them. (Try
spilling a drop of water on the fabric; if it beads up, it is water-resistant
and thus no good for dyeing.) If you can find them without such surface
finishes, even synthetic-fiber canvases should work well with acrylic fabric
paints such as Lumiere or Dye-Na-Flow. For the greatest durability without
heat-setting these paints, you can add a product called Jacquard AirSet, which
catalyzes the acrylic reaction without
heat.
Keds sells some shoes that are labeled as being stain-resistant: avoid these because they will not dye well. The fact that some are labeled as stain-resistant makes it seem hopeful that the others that are not so labeled are free of this dye-repelling finish. Their white twill shoes seem like a good bet.
(Please help support this web site. Thank you.)
Thursday, November 29, 2007
I have two all-white Chicago Cubs baseball caps that I'd like to dye a navy blue
Name: John
Message: I have looked through your site, but haven't found this question: I have two all-white Chicago Cubs baseball caps that I'd like to dye a navy blue. (Can you believe out of the hundreds of styles and colors, they don't have a simple all-navy cap? Anyway, I'm totally inept at these kinds of things. How can I dye the caps -- or is there someone who will do it, obviously for a fee?

What fiber are the caps made of? You can't know how–or whether—to dye anything unless you know what fiber it is made of. See my page, "About the Dyes".
Cotton hats can be easily dyed with a cool water fiber reactive dye such as Procion MX dye. I do not recommend all-purpose dye, such as Rit® dye or Tintex® dye, for cotton.

Polyester will not accept ordinary dyes, and I don't think you want to try using the special disperse dyes that are required for dyeing polyester.
Cotton/polyester blends will dye only the cotton portion. An 80%cotton/20% polyester blend will dye well with fiber reactive dye, but a 50%cotton/50%polyester blend will produce a pastel color, not so good for your goal of a dark navy blue, and lower percentages of cotton will dye more poorly still.

Nylon hats can be dyed with acid dyes, including all-purpose dyes, but they must be simmered, up to 185°F (85°C), in a cooking pot with the dye and a little vinegar. (Do not reuse the cooking pot for food after using it for dyeing.) Wool can be dyed exactly like nylon. The plastic parts sometimes used in baseball caps are usually nylon and will also dye with this method. Perhaps unfortunately, so will any nylon-thread embroidery or appliques.

An alternative to dye is the use of fabric paint, which will work on most synthetic fibers almost as well as on natural fibers. Jacquard Dye-Na-Flow [see advertisement below] is a fabric paint that flows like a dye. It will be impossible to get solid color results as smooth and even with any fabric paint as you can get with dye, and the results will tend to wear off much more quickly than a good fabric dye, but the advantage is that it will work on most fibers. However, fibers that have been treated with a stain-resistant or water-repellent coating will accept neither paint nor dyes.

Sorry, I do not know of anyone who will dye your baseball cap for you. It's not a common service these days. Please look at my page on "Where can I find someone to dye my clothing for me?". There are just a couple of companies that are willing to redye clothing from one color to another; whether they will be willing to dye your caps depends on their fiber content. I have seen various navy blue Chicago Cubs caps for sale, but they might not be the style you are looking for.
(Please help support this web site. Thank you.)
Advertisements
Message: I have looked through your site, but haven't found this question: I have two all-white Chicago Cubs baseball caps that I'd like to dye a navy blue. (Can you believe out of the hundreds of styles and colors, they don't have a simple all-navy cap? Anyway, I'm totally inept at these kinds of things. How can I dye the caps -- or is there someone who will do it, obviously for a fee?

What fiber are the caps made of? You can't know how–or whether—to dye anything unless you know what fiber it is made of. See my page, "About the Dyes".
Cotton hats can be easily dyed with a cool water fiber reactive dye such as Procion MX dye. I do not recommend all-purpose dye, such as Rit® dye or Tintex® dye, for cotton.

Polyester will not accept ordinary dyes, and I don't think you want to try using the special disperse dyes that are required for dyeing polyester.
Cotton/polyester blends will dye only the cotton portion. An 80%cotton/20% polyester blend will dye well with fiber reactive dye, but a 50%cotton/50%polyester blend will produce a pastel color, not so good for your goal of a dark navy blue, and lower percentages of cotton will dye more poorly still.

Nylon hats can be dyed with acid dyes, including all-purpose dyes, but they must be simmered, up to 185°F (85°C), in a cooking pot with the dye and a little vinegar. (Do not reuse the cooking pot for food after using it for dyeing.) Wool can be dyed exactly like nylon. The plastic parts sometimes used in baseball caps are usually nylon and will also dye with this method. Perhaps unfortunately, so will any nylon-thread embroidery or appliques.

An alternative to dye is the use of fabric paint, which will work on most synthetic fibers almost as well as on natural fibers. Jacquard Dye-Na-Flow [see advertisement below] is a fabric paint that flows like a dye. It will be impossible to get solid color results as smooth and even with any fabric paint as you can get with dye, and the results will tend to wear off much more quickly than a good fabric dye, but the advantage is that it will work on most fibers. However, fibers that have been treated with a stain-resistant or water-repellent coating will accept neither paint nor dyes.

Sorry, I do not know of anyone who will dye your baseball cap for you. It's not a common service these days. Please look at my page on "Where can I find someone to dye my clothing for me?". There are just a couple of companies that are willing to redye clothing from one color to another; whether they will be willing to dye your caps depends on their fiber content. I have seen various navy blue Chicago Cubs caps for sale, but they might not be the style you are looking for.
(Please help support this web site. Thank you.)
Advertisements
Wednesday, November 28, 2007
planning to dye a sweatshirt that has a silkscreened design. will the dyeing process cause any shrinkage in this kind of garment?
Name: Line
Message: Hi, I'm planning to dye a sweatshirt that has a silkscreened design on it. I think this will work because my sister in law has done other garments like this and I've loved them.
Silkscreened designs are unaffected by dyeing, in my experience, but you must keep the color light enough that the design does not simply seem to disappear, due to loss of contrast.
It's a white sweatshirt - Hanes® Heavyweight cotton poly blend. I have a large on order - will the dyeing process cause any shrinkage in this kind of garment? I hope I didn't miss this on the website - I did do a search.

There are two main classes of dyes for cotton: hot water
dyes, and cool water
dyes. Hot water dyes require nearly boiling water to perform their
best, which will, of course, cause as much cotton shrinkage as is possible. Hot
water dyes are widely available as Rit® Dye, Tintex® Easy Fabric Dye, DEKA® L dye,
etc. However, you should avoid hot water dyes when dyeing cotton clothing,
anyway. In particular, all-purpose
dyes perform poorly on cotton clothing, fading quickly in the laundry,
and (as a bonus) possibly ruining other clothing you wash the dyed garments
with, if you are not meticulous about sorting.
hot water
dyes, and cool water
dyes. Hot water dyes require nearly boiling water to perform their
best, which will, of course, cause as much cotton shrinkage as is possible. Hot
water dyes are widely available as Rit® Dye, Tintex® Easy Fabric Dye, DEKA® L dye,
etc. However, you should avoid hot water dyes when dyeing cotton clothing,
anyway. In particular, all-purpose
dyes perform poorly on cotton clothing, fading quickly in the laundry,
and (as a bonus) possibly ruining other clothing you wash the dyed garments
with, if you are not meticulous about sorting.
In contrast, a good cool water fiber reactive dye will not require boiling water. The most popular of fiber reactive dyes, Procion MX dye, can be used in water as cool as 70°F (21°C), which
will not shrink anything. It will also produce brighter, prettier results than
all-purpose dye can, and will last a hundred times longer in the laundry without
ever requiring you to wash it with similar colors. You will use soda ash to
fix the dye, instead of heat; this works only with fiber reactive dyes, never
with all-purpose dyes. Soda ash is a
common household chemical, found in washing soda and most laundry detergents;
you can buy it from the same company that sells you your Procion MX dye, or you
can get it from your local hardware store in the swimming pools supply
section.
which
will not shrink anything. It will also produce brighter, prettier results than
all-purpose dye can, and will last a hundred times longer in the laundry without
ever requiring you to wash it with similar colors. You will use soda ash to
fix the dye, instead of heat; this works only with fiber reactive dyes, never
with all-purpose dyes. Soda ash is a
common household chemical, found in washing soda and most laundry detergents;
you can buy it from the same company that sells you your Procion MX dye, or you
can get it from your local hardware store in the swimming pools supply
section.
However, the dyeing process absolutely requires a thorough washing afterwards, preferably washing in hot water of 140°F or higher, to remove excess unreacted dye. As you know, cotton does shrink when first washed, especially in hot water. If it is very important to you to maintain the pre-washing size of the shirt, you will have to wash out only in cool water. Cool water does not work nearly as efficiently in washing out fiber reactive dyes, so, if you do not use hot water for your washing out, you will have to be cautious with the garment, washing it separately or only with similar colors, until all of the excess dye has been removed, which could take many, many washings in cool water. It will work fine, though. You can do it. Just be aware that if the dyed clothing is washed in hot water for the first time even a year later, more dye will come out that did not come out in cool water. If you're ever going to wash in hot water, it's as well to do so right after dyeing, so that you know there is no unattached dye left to run in hot water.
As a lazy person, I prefer to buy clothing in the size that will fit even after machine washing and drying. This can save a lot of work over the lifetime of the garment. If I were you, I'd buy one size larger so that I could wash in hot water with impunity.
It is very important to note that while you can dye the cotton in your blended sweatshirt, you cannot dye the polyester. A
50% cotton/50% polyester blend will produce a nice pastel, if you use plenty of
dye. The same dyes will produce a brilliant color on a 100% cotton sweatshirt.
It is almost always preferable to dye a 100% cotton (or 100% rayon or 100% silk)
garment. 50% cotton is fine if you don't mind pastel colors, though. Polyester
will not accept any dyes that work on cotton; it will just stay white. (The
special
dye that will work on polyester requires that you boil the garment
with the dye for an hour, which will ruin many garments! Don't even bother to
try to dye the polyester fiber in your blended clothing.) Hanes does sell sweatshirts that are 100% cotton on the surface
and 80% cotton on the backing, which would be ideal for dyeing, as long as there
is no stain-resistant finish. 80% cotton generally dyes quite
well.
A
50% cotton/50% polyester blend will produce a nice pastel, if you use plenty of
dye. The same dyes will produce a brilliant color on a 100% cotton sweatshirt.
It is almost always preferable to dye a 100% cotton (or 100% rayon or 100% silk)
garment. 50% cotton is fine if you don't mind pastel colors, though. Polyester
will not accept any dyes that work on cotton; it will just stay white. (The
special
dye that will work on polyester requires that you boil the garment
with the dye for an hour, which will ruin many garments! Don't even bother to
try to dye the polyester fiber in your blended clothing.) Hanes does sell sweatshirts that are 100% cotton on the surface
and 80% cotton on the backing, which would be ideal for dyeing, as long as there
is no stain-resistant finish. 80% cotton generally dyes quite
well.
(Please help support this web site. Thank you.)
Message: Hi, I'm planning to dye a sweatshirt that has a silkscreened design on it. I think this will work because my sister in law has done other garments like this and I've loved them.
Silkscreened designs are unaffected by dyeing, in my experience, but you must keep the color light enough that the design does not simply seem to disappear, due to loss of contrast.
It's a white sweatshirt - Hanes® Heavyweight cotton poly blend. I have a large on order - will the dyeing process cause any shrinkage in this kind of garment? I hope I didn't miss this on the website - I did do a search.

There are two main classes of dyes for cotton:
 hot water
dyes, and cool water
dyes. Hot water dyes require nearly boiling water to perform their
best, which will, of course, cause as much cotton shrinkage as is possible. Hot
water dyes are widely available as Rit® Dye, Tintex® Easy Fabric Dye, DEKA® L dye,
etc. However, you should avoid hot water dyes when dyeing cotton clothing,
anyway. In particular, all-purpose
dyes perform poorly on cotton clothing, fading quickly in the laundry,
and (as a bonus) possibly ruining other clothing you wash the dyed garments
with, if you are not meticulous about sorting.
hot water
dyes, and cool water
dyes. Hot water dyes require nearly boiling water to perform their
best, which will, of course, cause as much cotton shrinkage as is possible. Hot
water dyes are widely available as Rit® Dye, Tintex® Easy Fabric Dye, DEKA® L dye,
etc. However, you should avoid hot water dyes when dyeing cotton clothing,
anyway. In particular, all-purpose
dyes perform poorly on cotton clothing, fading quickly in the laundry,
and (as a bonus) possibly ruining other clothing you wash the dyed garments
with, if you are not meticulous about sorting.In contrast, a good cool water fiber reactive dye will not require boiling water. The most popular of fiber reactive dyes, Procion MX dye, can be used in water as cool as 70°F (21°C),
 which
will not shrink anything. It will also produce brighter, prettier results than
all-purpose dye can, and will last a hundred times longer in the laundry without
ever requiring you to wash it with similar colors. You will use soda ash to
fix the dye, instead of heat; this works only with fiber reactive dyes, never
with all-purpose dyes. Soda ash is a
common household chemical, found in washing soda and most laundry detergents;
you can buy it from the same company that sells you your Procion MX dye, or you
can get it from your local hardware store in the swimming pools supply
section.
which
will not shrink anything. It will also produce brighter, prettier results than
all-purpose dye can, and will last a hundred times longer in the laundry without
ever requiring you to wash it with similar colors. You will use soda ash to
fix the dye, instead of heat; this works only with fiber reactive dyes, never
with all-purpose dyes. Soda ash is a
common household chemical, found in washing soda and most laundry detergents;
you can buy it from the same company that sells you your Procion MX dye, or you
can get it from your local hardware store in the swimming pools supply
section.However, the dyeing process absolutely requires a thorough washing afterwards, preferably washing in hot water of 140°F or higher, to remove excess unreacted dye. As you know, cotton does shrink when first washed, especially in hot water. If it is very important to you to maintain the pre-washing size of the shirt, you will have to wash out only in cool water. Cool water does not work nearly as efficiently in washing out fiber reactive dyes, so, if you do not use hot water for your washing out, you will have to be cautious with the garment, washing it separately or only with similar colors, until all of the excess dye has been removed, which could take many, many washings in cool water. It will work fine, though. You can do it. Just be aware that if the dyed clothing is washed in hot water for the first time even a year later, more dye will come out that did not come out in cool water. If you're ever going to wash in hot water, it's as well to do so right after dyeing, so that you know there is no unattached dye left to run in hot water.
As a lazy person, I prefer to buy clothing in the size that will fit even after machine washing and drying. This can save a lot of work over the lifetime of the garment. If I were you, I'd buy one size larger so that I could wash in hot water with impunity.
It is very important to note that while you can dye the cotton in your blended sweatshirt, you cannot dye the polyester.
 A
50% cotton/50% polyester blend will produce a nice pastel, if you use plenty of
dye. The same dyes will produce a brilliant color on a 100% cotton sweatshirt.
It is almost always preferable to dye a 100% cotton (or 100% rayon or 100% silk)
garment. 50% cotton is fine if you don't mind pastel colors, though. Polyester
will not accept any dyes that work on cotton; it will just stay white. (The
special
dye that will work on polyester requires that you boil the garment
with the dye for an hour, which will ruin many garments! Don't even bother to
try to dye the polyester fiber in your blended clothing.) Hanes does sell sweatshirts that are 100% cotton on the surface
and 80% cotton on the backing, which would be ideal for dyeing, as long as there
is no stain-resistant finish. 80% cotton generally dyes quite
well.
A
50% cotton/50% polyester blend will produce a nice pastel, if you use plenty of
dye. The same dyes will produce a brilliant color on a 100% cotton sweatshirt.
It is almost always preferable to dye a 100% cotton (or 100% rayon or 100% silk)
garment. 50% cotton is fine if you don't mind pastel colors, though. Polyester
will not accept any dyes that work on cotton; it will just stay white. (The
special
dye that will work on polyester requires that you boil the garment
with the dye for an hour, which will ruin many garments! Don't even bother to
try to dye the polyester fiber in your blended clothing.) Hanes does sell sweatshirts that are 100% cotton on the surface
and 80% cotton on the backing, which would be ideal for dyeing, as long as there
is no stain-resistant finish. 80% cotton generally dyes quite
well.(Please help support this web site. Thank you.)
Tuesday, November 27, 2007
How can I dye blue overalls to make them coral?
Name: lukas
Message: hi I ve got blue overhaulls and want to dye them Coral,what do I do,what two colors give Coral
Please help urgent
Regards

Sorry, it can't be done. The color coral does not contain any blue, so overdyeing blue overalls to create coral is impossible.
If you want coral overalls, and cannot find any in the store, you must first buy some overalls that are white, and made of 100% cotton. You could overdye a light yellow to make coral, or a light pink, but no other color. (Here's a link for buying white cotton overalls from Amazon.)
Buy some orange fiber reactive dye, such as Procion MX dye. (Avoid all-purpose dye, because it is hard to apply—you have to boil it for best results—and fades quickly in the laundry.) Along with your Procion MX dye, buy some salt and soda ash, and follow instructions for dyeing in the washing machine; see "How can I dye clothing or fabric in the washing machine?".
The trouble is that dye is transparent. It cannot cover up a darker color. Whatever color is already there in the garment will show through any dye that you apply.

If you have white overalls, it is easy to dye them coral. All you need is to dye them a pale shade of reddish orange. To produce a pale shade, you use a small quantity of dye. Although it is generally recommended that you use one teaspoon of this dye per pound of fabric to get a pale shade, for coral you should use less than this, perhaps 1/4 teaspoon to 1/2 teaspoon per pound of fabric. You can buy good Procion MX dye and soda ash from any of the suppliers listed on my page, Sources for Dyeing Supplies Around the World. (Here is a link for ordering orange Procion MX dye through Amazon, and another for buying soda ash.)
It might be possible to turn your blue overalls white by bleaching them, but it is likely that you will never be able to get out all of the color. Most likely, you will be able to produce a pale blue, which is good for overdyeing many colors, but no good at all for producing a nice clear coral.
The best colors for overdyeing blue overalls and blue jeans are all darker than the original color: navy blue, dark brown, deep purple, dark forest green, or black.
(Please help support this web site. Thank you.)
Message: hi I ve got blue overhaulls and want to dye them Coral,what do I do,what two colors give Coral
Please help urgent
Regards

Sorry, it can't be done. The color coral does not contain any blue, so overdyeing blue overalls to create coral is impossible.
If you want coral overalls, and cannot find any in the store, you must first buy some overalls that are white, and made of 100% cotton. You could overdye a light yellow to make coral, or a light pink, but no other color. (Here's a link for buying white cotton overalls from Amazon.)
Buy some orange fiber reactive dye, such as Procion MX dye. (Avoid all-purpose dye, because it is hard to apply—you have to boil it for best results—and fades quickly in the laundry.) Along with your Procion MX dye, buy some salt and soda ash, and follow instructions for dyeing in the washing machine; see "How can I dye clothing or fabric in the washing machine?".
The trouble is that dye is transparent. It cannot cover up a darker color. Whatever color is already there in the garment will show through any dye that you apply.

If you have white overalls, it is easy to dye them coral. All you need is to dye them a pale shade of reddish orange. To produce a pale shade, you use a small quantity of dye. Although it is generally recommended that you use one teaspoon of this dye per pound of fabric to get a pale shade, for coral you should use less than this, perhaps 1/4 teaspoon to 1/2 teaspoon per pound of fabric. You can buy good Procion MX dye and soda ash from any of the suppliers listed on my page, Sources for Dyeing Supplies Around the World. (Here is a link for ordering orange Procion MX dye through Amazon, and another for buying soda ash.)
It might be possible to turn your blue overalls white by bleaching them, but it is likely that you will never be able to get out all of the color. Most likely, you will be able to produce a pale blue, which is good for overdyeing many colors, but no good at all for producing a nice clear coral.
The best colors for overdyeing blue overalls and blue jeans are all darker than the original color: navy blue, dark brown, deep purple, dark forest green, or black.
(Please help support this web site. Thank you.)
Monday, November 26, 2007
will the cold prevent the dyes working?
Name: Helen Conway (downthewell.blogspot.com)
Message: I want to try hand dyeing a gradation of fabrics for quilting. I think that Cold water Dylon is the easiest for me to get hold of here in the UK. However, I am concerned about temperature. I have a tiny kitchen and will have to do it all out in the garage over the Christmas period which in the UK translates to just above freezing temperatures. I can wear layers but will the cold prevent the dyes working?

Yes, it will. It's good that you're thinking about this in advance. Dylon Cold Water Dye is a fiber reactive dye that does not require nearly as much warmth as, say, an all-purpose dye (which should be boiled with the fabric), but it does require some. Most of the colors in Dylon Cold Water Dye are Procion MX dyes, though a few are Drimarene K or Remazol type dyes. (See the post, "more about Dylon Cold Water Dyes ", in the Dye Forum on my site). The minimum temperature requirement is roughly 21°C (70°F), for dyes left to react with fabric overnight or longer; warmer temperatures work fine, too, and reduce the required amount of time. The reaction between fiber reactive dye and fabric can take place only in the presence of moisture, a high-pH chemical such as soda ash (also known as "Dylon Cold Fix"), and adequate warmth.
There are many different ways to heat up your dye reactions. If you're doing direct dye application, the simplest is to wrap them up so that you can safely take them into a warmer part of the house overnight. You can wrap each piece with plastic wrap, to prevent one part of the fabric from touching another when you roll it up, and then place the whole roll into a plastic bag, and carry the wrapped bundles in plastic buckets or boxes.

If you are dyeing in small buckets, you can fill your sink, or a large insulated cooler, with very hot tap water, and rest your buckets in there. (Don't fill the cooler so full of water that the buckets float; they might tip over, which would be a disaster.) The insulated cooler is ideal because it will hold the heat for hours. If you are dyeing in plastic bags, you can place several in each of a number of plastic buckets or bowls to heat in the same way. Alternatively, you can double-bag them in the sturdier zipper-top plastic bags used for freezer storage, and float those in the warm or hot water.

If you're working on a table, you can place an electric heating blanket or waterproof heated mattress pad (preferably one plugged into a ground fault circuit interrupter!) on the table, then cover that with a sturdy plastic sheet or tablecloth, and lay out your work on top of that, or do the reverse, laying your work out on the table, covering that with a reliable layer of sturdy thin plastic, with the electric blanket on top of that.
Avoid Dylon® Multi Purpose dye. Like Rit® dye and Tintex® Easy Fabric Dye, it is an all-purpose
dye which does not produce very long-lasting results on cotton, and it
requires high heat (87°C) to attach as well as possible to the fabric.
Dylon Cold Dye, Dylon Machine Dye, and Dylon Permanent dye (the latter available
in North America) all perform much better, since they are fiber reactive
dyes.
dye. Like Rit® dye and Tintex® Easy Fabric Dye, it is an all-purpose
dye which does not produce very long-lasting results on cotton, and it
requires high heat (87°C) to attach as well as possible to the fabric.
Dylon Cold Dye, Dylon Machine Dye, and Dylon Permanent dye (the latter available
in North America) all perform much better, since they are fiber reactive
dyes.
Dylon Cold Water dyes bond well to cotton fabric, since they are mostly Procion MX dyes, but they can be frustrating to work with, since the pure unmixed Procion MX dyes are not supplied in this line, but only mixtures of dye colors, designed to meet current fashion standards. There are no ideal primary colors in the Dylon Cold Dye line for mixing your own colors, because all have had some other color of dye added, which will cause your mixtures to be duller in color. Also, the tiny 5-gram tins are really very expensive, considering how little they will dye, only about 200 grams of fabric per tin, and only 100 grams for black. You will have to buy a great many of them if you are planning on doing much dyeing.
There are better dyes that you can buy, instead of Dylon dyes, if
you are going to be mixing your colors or doing color gradations. In the UK,
there are several good sources for buying fiber reactive dye by mail-order. See
my page of Sources
for Dyeing Supplies Around the World, scrolling down to the
section on Europe. For example, Fibrecrafts/George Weil sells Procion MX dyes
for £8.40 for 50 grams of dye, equivalent to at least ten of the tiny tins
of Dylon Cold, which each cost about £1.89. (It may be a better value
still, since the Dylon dye powders are likely to be more dilute than the Procion
MX dye powders sold by Fibrecrafts.) Fibrecrafts sells a good range of the pure unmixed
single-hue Procion MX dyes, which many dyers find to be much more
satisfying for use in color mixing and color gradations. I recommend that, at
minimum, you purchase lemon yellow, vibrant magenta or brilliant pink, brilliant
turquoise, and black; I recommend also that you get some golden yellow and
indigo navy. Also consider the other good mail-order suppliers listed on my page
of Sources
for Dyeing Supplies Around the World. You can buy soda ash
locally or from your mail-order dye supplier for much less money than Dylon Cold
Fix, as well, although they are identical substances.
if
you are going to be mixing your colors or doing color gradations. In the UK,
there are several good sources for buying fiber reactive dye by mail-order. See
my page of Sources
for Dyeing Supplies Around the World, scrolling down to the
section on Europe. For example, Fibrecrafts/George Weil sells Procion MX dyes
for £8.40 for 50 grams of dye, equivalent to at least ten of the tiny tins
of Dylon Cold, which each cost about £1.89. (It may be a better value
still, since the Dylon dye powders are likely to be more dilute than the Procion
MX dye powders sold by Fibrecrafts.) Fibrecrafts sells a good range of the pure unmixed
single-hue Procion MX dyes, which many dyers find to be much more
satisfying for use in color mixing and color gradations. I recommend that, at
minimum, you purchase lemon yellow, vibrant magenta or brilliant pink, brilliant
turquoise, and black; I recommend also that you get some golden yellow and
indigo navy. Also consider the other good mail-order suppliers listed on my page
of Sources
for Dyeing Supplies Around the World. You can buy soda ash
locally or from your mail-order dye supplier for much less money than Dylon Cold
Fix, as well, although they are identical substances.
(Please help support this web site. Thank you.)
Message: I want to try hand dyeing a gradation of fabrics for quilting. I think that Cold water Dylon is the easiest for me to get hold of here in the UK. However, I am concerned about temperature. I have a tiny kitchen and will have to do it all out in the garage over the Christmas period which in the UK translates to just above freezing temperatures. I can wear layers but will the cold prevent the dyes working?

Yes, it will. It's good that you're thinking about this in advance. Dylon Cold Water Dye is a fiber reactive dye that does not require nearly as much warmth as, say, an all-purpose dye (which should be boiled with the fabric), but it does require some. Most of the colors in Dylon Cold Water Dye are Procion MX dyes, though a few are Drimarene K or Remazol type dyes. (See the post, "more about Dylon Cold Water Dyes ", in the Dye Forum on my site). The minimum temperature requirement is roughly 21°C (70°F), for dyes left to react with fabric overnight or longer; warmer temperatures work fine, too, and reduce the required amount of time. The reaction between fiber reactive dye and fabric can take place only in the presence of moisture, a high-pH chemical such as soda ash (also known as "Dylon Cold Fix"), and adequate warmth.
There are many different ways to heat up your dye reactions. If you're doing direct dye application, the simplest is to wrap them up so that you can safely take them into a warmer part of the house overnight. You can wrap each piece with plastic wrap, to prevent one part of the fabric from touching another when you roll it up, and then place the whole roll into a plastic bag, and carry the wrapped bundles in plastic buckets or boxes.

If you are dyeing in small buckets, you can fill your sink, or a large insulated cooler, with very hot tap water, and rest your buckets in there. (Don't fill the cooler so full of water that the buckets float; they might tip over, which would be a disaster.) The insulated cooler is ideal because it will hold the heat for hours. If you are dyeing in plastic bags, you can place several in each of a number of plastic buckets or bowls to heat in the same way. Alternatively, you can double-bag them in the sturdier zipper-top plastic bags used for freezer storage, and float those in the warm or hot water.

If you're working on a table, you can place an electric heating blanket or waterproof heated mattress pad (preferably one plugged into a ground fault circuit interrupter!) on the table, then cover that with a sturdy plastic sheet or tablecloth, and lay out your work on top of that, or do the reverse, laying your work out on the table, covering that with a reliable layer of sturdy thin plastic, with the electric blanket on top of that.
Avoid Dylon® Multi Purpose
 dye. Like Rit® dye and Tintex® Easy Fabric Dye, it is an all-purpose
dye which does not produce very long-lasting results on cotton, and it
requires high heat (87°C) to attach as well as possible to the fabric.
Dylon Cold Dye, Dylon Machine Dye, and Dylon Permanent dye (the latter available
in North America) all perform much better, since they are fiber reactive
dyes.
dye. Like Rit® dye and Tintex® Easy Fabric Dye, it is an all-purpose
dye which does not produce very long-lasting results on cotton, and it
requires high heat (87°C) to attach as well as possible to the fabric.
Dylon Cold Dye, Dylon Machine Dye, and Dylon Permanent dye (the latter available
in North America) all perform much better, since they are fiber reactive
dyes.Dylon Cold Water dyes bond well to cotton fabric, since they are mostly Procion MX dyes, but they can be frustrating to work with, since the pure unmixed Procion MX dyes are not supplied in this line, but only mixtures of dye colors, designed to meet current fashion standards. There are no ideal primary colors in the Dylon Cold Dye line for mixing your own colors, because all have had some other color of dye added, which will cause your mixtures to be duller in color. Also, the tiny 5-gram tins are really very expensive, considering how little they will dye, only about 200 grams of fabric per tin, and only 100 grams for black. You will have to buy a great many of them if you are planning on doing much dyeing.
There are better dyes that you can buy, instead of Dylon dyes,
 if
you are going to be mixing your colors or doing color gradations. In the UK,
there are several good sources for buying fiber reactive dye by mail-order. See
my page of Sources
for Dyeing Supplies Around the World, scrolling down to the
section on Europe. For example, Fibrecrafts/George Weil sells Procion MX dyes
for £8.40 for 50 grams of dye, equivalent to at least ten of the tiny tins
of Dylon Cold, which each cost about £1.89. (It may be a better value
still, since the Dylon dye powders are likely to be more dilute than the Procion
MX dye powders sold by Fibrecrafts.) Fibrecrafts sells a good range of the pure unmixed
single-hue Procion MX dyes, which many dyers find to be much more
satisfying for use in color mixing and color gradations. I recommend that, at
minimum, you purchase lemon yellow, vibrant magenta or brilliant pink, brilliant
turquoise, and black; I recommend also that you get some golden yellow and
indigo navy. Also consider the other good mail-order suppliers listed on my page
of Sources
for Dyeing Supplies Around the World. You can buy soda ash
locally or from your mail-order dye supplier for much less money than Dylon Cold
Fix, as well, although they are identical substances.
if
you are going to be mixing your colors or doing color gradations. In the UK,
there are several good sources for buying fiber reactive dye by mail-order. See
my page of Sources
for Dyeing Supplies Around the World, scrolling down to the
section on Europe. For example, Fibrecrafts/George Weil sells Procion MX dyes
for £8.40 for 50 grams of dye, equivalent to at least ten of the tiny tins
of Dylon Cold, which each cost about £1.89. (It may be a better value
still, since the Dylon dye powders are likely to be more dilute than the Procion
MX dye powders sold by Fibrecrafts.) Fibrecrafts sells a good range of the pure unmixed
single-hue Procion MX dyes, which many dyers find to be much more
satisfying for use in color mixing and color gradations. I recommend that, at
minimum, you purchase lemon yellow, vibrant magenta or brilliant pink, brilliant
turquoise, and black; I recommend also that you get some golden yellow and
indigo navy. Also consider the other good mail-order suppliers listed on my page
of Sources
for Dyeing Supplies Around the World. You can buy soda ash
locally or from your mail-order dye supplier for much less money than Dylon Cold
Fix, as well, although they are identical substances.(Please help support this web site. Thank you.)
Sunday, November 25, 2007
My tub is now a nice shade of blue and scrubbing it with Ajax is NOT helping. Any ideas on how I can get it off
Name: Elisheva
Message: My work space right now is my bathroom...My tub is now a nice shade of blue and scrubbing it with Ajax is NOT helping. Any ideas on how I can get it off?
What is "it" that you need to get off? Rit or Deka all-purpose dye, fabric paint, Procion MX dye, or what? Did you immersion-dye in the bathtub, or just have some splashing from your dye bucket?
oops, sorry I forgot that detail. this is MX dye that got all over due to me washing out the shelf I was using to dye the items, spilling out dye at the bottom of my "project box" AND washing that out frequently...and last but not least, taking off rubber brands and rinsing out each and every item before I threw it into the wash (and that was about 150 things...)
Wow, that's not a problem I've ever seen before. Wherever I've lived, I've only ever had old-fashioned bathtubs with a porcelain finish over cast iron. Until the surface gloss gets worn off (and my current bathtub is sixty years old and still plenty glossy), the porcelain absolutely does not stain with Procion MX dyes. Soap scum or dirt on the surface of the tub will pick up dye, but that's easily removed, obviously not your problem since you've already tried scouring powder without success.
Presumably your bathtub is made of a more "modern", cheaper material, unless it's porcelain with the glossy surface all worn off, or a tub that was refinished some time ago. It might be made of fiberglass, or acrylic. Neither of these will react with Procion MX dye, the way that cellulose (cotton, rayon, or wood) or protein (wool or silk) might do. What I'm afraid of is that one of these might have a more porous nature. If it has actually absorbed the dye into itself, it will be much harder to remove—as you have already observed.
The first step is to scrub as thoroughly as possible,
using a bleach-containing powder, such as your Ajax. Since that has failed,
you'll want to try something else.
possible,
using a bleach-containing powder, such as your Ajax. Since that has failed,
you'll want to try something else.
If your bathtub has been refinished, or if it is made of fiberglass or acrylic, you may need to stick to whatever cleaners are recommended by the manufacturer. If you are renting, the only way to find out if this is the case is to ask the apartment management.

If the material your tub is made of does not react badly to chlorine bleach, you could probably use that to break up and decolorize the dye molecules. However, I've read that some bathtubs develop an ugly reddish-brown stain when treated with bleach. You should test a small area before treating much of your tub with bleach. (According to the Clorox company, you can use "Clorox 2 Bleach for Colors", which is a perborate-based laundry cleaner that liberates peroxide, to remove reddish-brown bathtub stains produced by bleach-containing cleansers.)
Be aware that, for all its familiarity, chlorine
bleach
chlorine
bleach
 (active
ingredient: hypochlorite) is a dangerous, toxic chemical. Wear reusable
housecleaning rubber gloves, rather than the thin disposable latex gloves that
often get holes in them. Use fans to provide good ventilation. If too much
exposure to the fumes from the bleach is inevitable, wear a properly-fitting
respirator, outfitted with with acid gas cartridges, to protect you from
breathing the bleach
fumes.
(active
ingredient: hypochlorite) is a dangerous, toxic chemical. Wear reusable
housecleaning rubber gloves, rather than the thin disposable latex gloves that
often get holes in them. Use fans to provide good ventilation. If too much
exposure to the fumes from the bleach is inevitable, wear a properly-fitting
respirator, outfitted with with acid gas cartridges, to protect you from
breathing the bleach
fumes.

How to keep the bleach on the surface long enough to fully act? You might put a rag on the surface and dampen it with liquid bleach, or for small areas you could use the thickened bleach in a Clorox Bleach Pen, or for larger areas you could apply a dishwasher detergent whose label claims "with bleach" and whose ingredients include hypochlorite. Be very careful to avoid any situation in which the bleach might become mixed with any other cleaner, which might contain ammonia (producing deadly chloramine gas), or with any acid such as vinegar or toilet cleaner (producing deadly chlorine gas). Leave your bleach to act for some time, then rinse thoroughly. Be aware that this treatment can damage the grout between floor tiles.
(Please help support this web site. Thank you.)
Message: My work space right now is my bathroom...My tub is now a nice shade of blue and scrubbing it with Ajax is NOT helping. Any ideas on how I can get it off?
What is "it" that you need to get off? Rit or Deka all-purpose dye, fabric paint, Procion MX dye, or what? Did you immersion-dye in the bathtub, or just have some splashing from your dye bucket?
oops, sorry I forgot that detail. this is MX dye that got all over due to me washing out the shelf I was using to dye the items, spilling out dye at the bottom of my "project box" AND washing that out frequently...and last but not least, taking off rubber brands and rinsing out each and every item before I threw it into the wash (and that was about 150 things...)
Wow, that's not a problem I've ever seen before. Wherever I've lived, I've only ever had old-fashioned bathtubs with a porcelain finish over cast iron. Until the surface gloss gets worn off (and my current bathtub is sixty years old and still plenty glossy), the porcelain absolutely does not stain with Procion MX dyes. Soap scum or dirt on the surface of the tub will pick up dye, but that's easily removed, obviously not your problem since you've already tried scouring powder without success.
Presumably your bathtub is made of a more "modern", cheaper material, unless it's porcelain with the glossy surface all worn off, or a tub that was refinished some time ago. It might be made of fiberglass, or acrylic. Neither of these will react with Procion MX dye, the way that cellulose (cotton, rayon, or wood) or protein (wool or silk) might do. What I'm afraid of is that one of these might have a more porous nature. If it has actually absorbed the dye into itself, it will be much harder to remove—as you have already observed.
The first step is to scrub as thoroughly as
 possible,
using a bleach-containing powder, such as your Ajax. Since that has failed,
you'll want to try something else.
possible,
using a bleach-containing powder, such as your Ajax. Since that has failed,
you'll want to try something else.If your bathtub has been refinished, or if it is made of fiberglass or acrylic, you may need to stick to whatever cleaners are recommended by the manufacturer. If you are renting, the only way to find out if this is the case is to ask the apartment management.

If the material your tub is made of does not react badly to chlorine bleach, you could probably use that to break up and decolorize the dye molecules. However, I've read that some bathtubs develop an ugly reddish-brown stain when treated with bleach. You should test a small area before treating much of your tub with bleach. (According to the Clorox company, you can use "Clorox 2 Bleach for Colors", which is a perborate-based laundry cleaner that liberates peroxide, to remove reddish-brown bathtub stains produced by bleach-containing cleansers.)
Be aware that, for all its familiarity,
 chlorine
bleach
chlorine
bleach
 (active
ingredient: hypochlorite) is a dangerous, toxic chemical. Wear reusable
housecleaning rubber gloves, rather than the thin disposable latex gloves that
often get holes in them. Use fans to provide good ventilation. If too much
exposure to the fumes from the bleach is inevitable, wear a properly-fitting
respirator, outfitted with with acid gas cartridges, to protect you from
breathing the bleach
fumes.
(active
ingredient: hypochlorite) is a dangerous, toxic chemical. Wear reusable
housecleaning rubber gloves, rather than the thin disposable latex gloves that
often get holes in them. Use fans to provide good ventilation. If too much
exposure to the fumes from the bleach is inevitable, wear a properly-fitting
respirator, outfitted with with acid gas cartridges, to protect you from
breathing the bleach
fumes. 
How to keep the bleach on the surface long enough to fully act? You might put a rag on the surface and dampen it with liquid bleach, or for small areas you could use the thickened bleach in a Clorox Bleach Pen, or for larger areas you could apply a dishwasher detergent whose label claims "with bleach" and whose ingredients include hypochlorite. Be very careful to avoid any situation in which the bleach might become mixed with any other cleaner, which might contain ammonia (producing deadly chloramine gas), or with any acid such as vinegar or toilet cleaner (producing deadly chlorine gas). Leave your bleach to act for some time, then rinse thoroughly. Be aware that this treatment can damage the grout between floor tiles.
(Please help support this web site. Thank you.)
Saturday, November 24, 2007
I received a bleach stain on my beige cotton sweater. I would like to dye the entire sweater. Can this be done?
Name: Lisa
Message: I received a bleach stain on my beige cotton sweater. I would like to dye the entire sweater. Can this be done? If I dye the sweater, I want the entire sweater to dye, including the bleach stain.
This is a frequently asked question on this site: Help! I ruined clothing by spattering bleach. How can I fix it?
The problem with dyeing the sweater to cover it up is that dye is transparent,
so the bleached spot will inevitably come out a lighter color than the rest of
the sweater. If the region affected is small, you can try covering it with a
fabric
marker , and then if necessary overdyeing a darker color, but if the
stain is large, the only way to really cover it up would be to not even try to
get a perfectly smooth solid color, but instead try low water
immersion dyeing or even tie-dyeing to
minimize its effect.
transparent,
so the bleached spot will inevitably come out a lighter color than the rest of
the sweater. If the region affected is small, you can try covering it with a
fabric
marker , and then if necessary overdyeing a darker color, but if the
stain is large, the only way to really cover it up would be to not even try to
get a perfectly smooth solid color, but instead try low water
immersion dyeing or even tie-dyeing to
minimize its effect.
(Please help support this web site. Thank you.)
Message: I received a bleach stain on my beige cotton sweater. I would like to dye the entire sweater. Can this be done? If I dye the sweater, I want the entire sweater to dye, including the bleach stain.
This is a frequently asked question on this site: Help! I ruined clothing by spattering bleach. How can I fix it?
The problem with dyeing the sweater to cover it up is that dye is
 transparent,
so the bleached spot will inevitably come out a lighter color than the rest of
the sweater. If the region affected is small, you can try covering it with a
fabric
marker , and then if necessary overdyeing a darker color, but if the
stain is large, the only way to really cover it up would be to not even try to
get a perfectly smooth solid color, but instead try low water
immersion dyeing or even tie-dyeing to
minimize its effect.
transparent,
so the bleached spot will inevitably come out a lighter color than the rest of
the sweater. If the region affected is small, you can try covering it with a
fabric
marker , and then if necessary overdyeing a darker color, but if the
stain is large, the only way to really cover it up would be to not even try to
get a perfectly smooth solid color, but instead try low water
immersion dyeing or even tie-dyeing to
minimize its effect.(Please help support this web site. Thank you.)
Friday, November 23, 2007
toxicity and environmental damage associated with logwood and other natural dyes
Name: Deborah
Message: I've just started to experiment with natural dyeing. I've done some research about the toxicity of mordants and dye stuffs, but I have not heard about the problem of hematein derived from logwood. Is this also an environmental problem? Is there a source that you can recommend for more information about toxicity and/or environmental damage caused by natural dyeing? I have already read Jim Lile's and Jenny Dean's books, as well as the information from aurorasilk.com.
Books and websites about natural dyes tend not to consider toxicity as a topic. Many neglect or groundlessly minimize the dangers of even such serious health hazards such as chrome, copper, and tin mordants. In order to research this topic, it is necessary to search for specific information from other sources about chemicals. If you know the name of the active principle in the dye, in this case hematein as the active ingredient in logwood, you can often find further information.
Hemetein, the oxidized form
of hematoxylin (Colour Index Natural Black 1), in addition to its use as a
textile dye, is an important stain for use in cytology. Here is a link to
an MSDS for hematein, which identifies it as having
a health hazard rating of 2 (where 0 is harmless and 4 is the maximum). It says,
among other
things,
form
of hematoxylin (Colour Index Natural Black 1), in addition to its use as a
textile dye, is an important stain for use in cytology. Here is a link to
an MSDS for hematein, which identifies it as having
a health hazard rating of 2 (where 0 is harmless and 4 is the maximum). It says,
among other
things,

Unfortunately, the MSDS says little about ecological hazards. Whatever harm may be posed ecologically by this material, it must be vanishingly small in comparison to its use in its heyday, when logwood plus iron was the most reliable black dye commercially available, before the introduction of modern synthetic dyes. Clear-cutting of Brazilian forests for logwood production undoubtedly resulted in much environmental damage. I have no idea whether logwood harvesting is currently practiced in an environmentally harmful way. [Followup: "Logwood from Aurora Silk is supposed to come from an environmentally and socially sustainable project."]
As long as you are steering clear of dangerous mordants such as hexavalent chromium (potassium dichromate, a
known human carcinogen) or tin, you will probably be safe enough with quite
ordinary precautions. Always wear a properly-fitting dust mask or respirator
when working with dye powder; never reuse dyepots or other implements for food;
wear a lab coat or apron to protect clothing, goggles to protect the eyes if
splashing is possible, and always wear reliable gloves while dyeing. (Some
advise against the use of a dust mask, recommending only a NIOSH-approved toxic
dust respirator.) I do not feel that these precautions are sufficient for
working with chrome mordants, which are far too unsafe, in my opinion, for use
in the home dye studio. Hematein is not nearly as hazardous as a chrome mordant.
Prechromed synthetic dyes are safer than chrome mordant because smaller
quantities of chromium are involved, and (more importantly) because they are in
the much safer trivalent form of chromium.
a
known human carcinogen) or tin, you will probably be safe enough with quite
ordinary precautions. Always wear a properly-fitting dust mask or respirator
when working with dye powder; never reuse dyepots or other implements for food;
wear a lab coat or apron to protect clothing, goggles to protect the eyes if
splashing is possible, and always wear reliable gloves while dyeing. (Some
advise against the use of a dust mask, recommending only a NIOSH-approved toxic
dust respirator.) I do not feel that these precautions are sufficient for
working with chrome mordants, which are far too unsafe, in my opinion, for use
in the home dye studio. Hematein is not nearly as hazardous as a chrome mordant.
Prechromed synthetic dyes are safer than chrome mordant because smaller
quantities of chromium are involved, and (more importantly) because they are in
the much safer trivalent form of chromium.
Many dyers believe all natural dyes to be completely safe, but in fact this is often not the case. Besides hematein, there are other toxic natural dyes as well. For example, the dyes alizarin and purpurin, derived from madder, are associated with kidney damage in animal experiments. Lily-of-the-valley, sometimes used for a pale green, is certainly toxic, as is bloodroot. The leaves of rhubarb contain toxic oxalic acid. Even relatively non-toxic dyes such as cochineal are associated with allergies, especially among the workers who prepare them. However, I think that all of these are unlikely to be a problem for the careful dyer, who takes appropriate precautions and does not do anything that will result in breathing, eating, or otherwise consuming dyestuffs.
(Please help support this web site. Thank you.)
Message: I've just started to experiment with natural dyeing. I've done some research about the toxicity of mordants and dye stuffs, but I have not heard about the problem of hematein derived from logwood. Is this also an environmental problem? Is there a source that you can recommend for more information about toxicity and/or environmental damage caused by natural dyeing? I have already read Jim Lile's and Jenny Dean's books, as well as the information from aurorasilk.com.
Books and websites about natural dyes tend not to consider toxicity as a topic. Many neglect or groundlessly minimize the dangers of even such serious health hazards such as chrome, copper, and tin mordants. In order to research this topic, it is necessary to search for specific information from other sources about chemicals. If you know the name of the active principle in the dye, in this case hematein as the active ingredient in logwood, you can often find further information.
Hemetein, the oxidized
 form
of hematoxylin (Colour Index Natural Black 1), in addition to its use as a
textile dye, is an important stain for use in cytology. Here is a link to
an MSDS for hematein, which identifies it as having
a health hazard rating of 2 (where 0 is harmless and 4 is the maximum). It says,
among other
things,
form
of hematoxylin (Colour Index Natural Black 1), in addition to its use as a
textile dye, is an important stain for use in cytology. Here is a link to
an MSDS for hematein, which identifies it as having
a health hazard rating of 2 (where 0 is harmless and 4 is the maximum). It says,
among other
things,"After contact with skin, wash immediately with plenty of water. Gently and thoroughly wash the contaminated skin with running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. Cover the irritated skin with an emollient. If irritation persists, seek medical attention. Wash contaminated clothing before reusing."

Unfortunately, the MSDS says little about ecological hazards. Whatever harm may be posed ecologically by this material, it must be vanishingly small in comparison to its use in its heyday, when logwood plus iron was the most reliable black dye commercially available, before the introduction of modern synthetic dyes. Clear-cutting of Brazilian forests for logwood production undoubtedly resulted in much environmental damage. I have no idea whether logwood harvesting is currently practiced in an environmentally harmful way. [Followup: "Logwood from Aurora Silk is supposed to come from an environmentally and socially sustainable project."]
As long as you are steering clear of dangerous mordants such as hexavalent chromium (potassium dichromate,
 a
known human carcinogen) or tin, you will probably be safe enough with quite
ordinary precautions. Always wear a properly-fitting dust mask or respirator
when working with dye powder; never reuse dyepots or other implements for food;
wear a lab coat or apron to protect clothing, goggles to protect the eyes if
splashing is possible, and always wear reliable gloves while dyeing. (Some
advise against the use of a dust mask, recommending only a NIOSH-approved toxic
dust respirator.) I do not feel that these precautions are sufficient for
working with chrome mordants, which are far too unsafe, in my opinion, for use
in the home dye studio. Hematein is not nearly as hazardous as a chrome mordant.
Prechromed synthetic dyes are safer than chrome mordant because smaller
quantities of chromium are involved, and (more importantly) because they are in
the much safer trivalent form of chromium.
a
known human carcinogen) or tin, you will probably be safe enough with quite
ordinary precautions. Always wear a properly-fitting dust mask or respirator
when working with dye powder; never reuse dyepots or other implements for food;
wear a lab coat or apron to protect clothing, goggles to protect the eyes if
splashing is possible, and always wear reliable gloves while dyeing. (Some
advise against the use of a dust mask, recommending only a NIOSH-approved toxic
dust respirator.) I do not feel that these precautions are sufficient for
working with chrome mordants, which are far too unsafe, in my opinion, for use
in the home dye studio. Hematein is not nearly as hazardous as a chrome mordant.
Prechromed synthetic dyes are safer than chrome mordant because smaller
quantities of chromium are involved, and (more importantly) because they are in
the much safer trivalent form of chromium.Many dyers believe all natural dyes to be completely safe, but in fact this is often not the case. Besides hematein, there are other toxic natural dyes as well. For example, the dyes alizarin and purpurin, derived from madder, are associated with kidney damage in animal experiments. Lily-of-the-valley, sometimes used for a pale green, is certainly toxic, as is bloodroot. The leaves of rhubarb contain toxic oxalic acid. Even relatively non-toxic dyes such as cochineal are associated with allergies, especially among the workers who prepare them. However, I think that all of these are unlikely to be a problem for the careful dyer, who takes appropriate precautions and does not do anything that will result in breathing, eating, or otherwise consuming dyestuffs.
(Please help support this web site. Thank you.)
Thursday, November 22, 2007
backstaining of dye in batik, and unwanted pale colors in batik
Name: Mateo
Message: Hi,
I am a batik "artist" and I am writing as you you have one of the most informative sites that I have been to. The question I have for you is about staining of the white or waxed areas with other colors and the other I have for you you is about dharma's Navy blue.
1. Have you had problems with the overdye staining the white areas and also the pre dyed areas of the fabric leaving the entire batik dull and and dirty looking? If so is there a way to avoid this aside from adding more synthrapol to the boiling and final washing (yes I rinse all the shirts that I do but this has become an issue as of late, leaving me with many "dirty" or "old looking batiks").
There are two ways in which a fiber reactive dye such as Procion MX dye can back-stain fabric.
One is by having still-reactive dye in the fabric when you go to rinse it out. When this happens, the unreacted dye can get on the fabric in the wrong place and stain it
permanently by reacting with it. There is no way to solve this problem after it
happens, since the dye in the wrong places is as firmly attached as that in the
right places. The solution is to prevent this by making sure that the dye that
you have applied has fully reacted before you wash it out. Some of the dye will
have reacted with the fabric and become attached to it, while some will have
reacted with the water ("hydrolyzed") and will just have gone bad. Hydrolyzed
dye is not a threat because it cannot react. The easy way to make sure that all
your dye has reacted is to give it plenty of time; for example, if your dye
studio is at least 70°F (21°C), leave your dye reaction to run
overnight, while making sure (with either urea in the dye mixture, or plastic
over the fabric) that the dye stays moist on the fabric throughout the entire
reaction time.
happens, the unreacted dye can get on the fabric in the wrong place and stain it
permanently by reacting with it. There is no way to solve this problem after it
happens, since the dye in the wrong places is as firmly attached as that in the
right places. The solution is to prevent this by making sure that the dye that
you have applied has fully reacted before you wash it out. Some of the dye will
have reacted with the fabric and become attached to it, while some will have
reacted with the water ("hydrolyzed") and will just have gone bad. Hydrolyzed
dye is not a threat because it cannot react. The easy way to make sure that all
your dye has reacted is to give it plenty of time; for example, if your dye
studio is at least 70°F (21°C), leave your dye reaction to run
overnight, while making sure (with either urea in the dye mixture, or plastic
over the fabric) that the dye stays moist on the fabric throughout the entire
reaction time.
If your dye reaction is too cool, then it will not complete in the time alloted. You may need to take care to use warmer water, always, of course, remaining below the hot temperature at which the batik wax will soften. Water that is 90°F or 100°F (32°C or 38°C) will help speed the dye reaction without softening your wax. There are various ways to keep your dye reaction warm, but that is the subject of another article.
The other form of backstaining is caused by dye that has already hydrolyzed, so it cannot form the permanent covalent bond that holds Procion MX dye to the fabric under optimal conditions. However, the dye can still associate with the fabric, albeit more loosely, in much the same way that all-purpose dye associates with cotton. Once this has happened, the solution is HOT water. When you rinse out your dye after dyeing, you start with cool water to remove all dye auxiliaries such as soda ash or salt. After that, you should use hot water to remove the dye, at least 140°F (60°C) if possible, and soak the fabric in the hot water for a little while. Hotter water is more effective still at removing excess unattached dye. A fairly short washing in nearly-boiling water will remove excess unattached Procion MX dye very effectively. This should be happening already when you boil out your wax after you are done batiking, shouldn't it?
There can be added complications that make it
harder to wash out dye. If the water is hard, the hardness minerals can form
complexes with the dyes which are much more difficult to wash out. In this case,
add a small amount of sodium hexametaphosphate to the water for every step of
dyeing, even the dye wash-out stage if necessary. See my FAQ on "We have very hard water.
Should I use distilled or spring water instead of tap
water?".
it
harder to wash out dye. If the water is hard, the hardness minerals can form
complexes with the dyes which are much more difficult to wash out. In this case,
add a small amount of sodium hexametaphosphate to the water for every step of
dyeing, even the dye wash-out stage if necessary. See my FAQ on "We have very hard water.
Should I use distilled or spring water instead of tap
water?".
Another complication that can make dye appear to
be difficult to wash out is the presence of sizing in the fabric. This should
not be a problem if you are starting with PFD ("prepared for dyeing") fabric. If
there is starch in the fabric, a drop of tincture of iodine, placed on the
apparently clean fabric, will reveal the presence of the starch by turning blue.
Starch is extraordinarily difficult to full remove from fabric. Even boiling
with soda ash will not do it. Starch in the fabric will react with the dye in
just the same way as the cellulose in the fabric, but will gradually wash out,
giving the appearance of poor washfastness. Never dye fabric that has been sized
with starch, and beware of other sizings as well, which may be equally
problematic for one reason or another.
to
be difficult to wash out is the presence of sizing in the fabric. This should
not be a problem if you are starting with PFD ("prepared for dyeing") fabric. If
there is starch in the fabric, a drop of tincture of iodine, placed on the
apparently clean fabric, will reveal the presence of the starch by turning blue.
Starch is extraordinarily difficult to full remove from fabric. Even boiling
with soda ash will not do it. Starch in the fabric will react with the dye in
just the same way as the cellulose in the fabric, but will gradually wash out,
giving the appearance of poor washfastness. Never dye fabric that has been sized
with starch, and beware of other sizings as well, which may be equally
problematic for one reason or another.
A more common complication that leads to difficult wash-out of excess dye is failure to use a cool rinse before washing in hot water. The presence of auxiliary chemicals such as salt or soda ash, when you put the fabric in hot water, may encourage the dye to form those loose associations that are like those of the direct dye in all-purpose dye mixtures. These associations makes washing-out so much more difficult! Never do you very first rinse of Procion MX or other fiber reactive dye in hot water. Always use cool water first to remove all auxiliary chemicals.
The other problem that I have been having in the past 6 months is the navy blue from Dharma not being as deep as I would like to have it (I do many night batiks and this is a color that I use to set off the rest of the colors) when the boiling and washing is done I am finding that the color is washed out and there is webbing where the wax has cracked leaving the color pale and almost bleached out.
We have taken to filling buckets with water and letting it sit over night to eliminate any of the chlorine in the water but it seems like this may be happening in the the rinse or boiling time....please let me know if there is anything that you think may help.
Much thanks
When your dye is failing to properly react with your fabric, which is what you are describing here, there are a number of different possible reasons. Number one is dye studio temperature: at what temperature is your dye reaction taking place, and for how long are you leaving it at this temperature? Procion MX type dyes react with the fabric very poorly below about 70°F (21°C). The warmer the dye reaction, the faster it goes.
Another possible cause of a poor dye reaction is bad fabric. If the fabric is sized with who-knows-what modern polymer surface treatment, or treated to make it permanent-press or stain-resistant, there's no telling what will happen. Of course, fabric that is not 100% cotton (or silk or rayon) is not going to react well. Occasionally fabric is mistakenly sold under the label of "100% cotton" when it is actually 50% polyester, which will not take dye well. I would advise you to use only PFD fabric from trustworthy sources for your batik fabric. Any cotton or rayon fabric purchased from Dharma Trading Company, Jacquard Fabrics, or TestFabrics Inc., for example, should be fine. You just need to use PFD cotton or rayon fabric.

Sometimes a problem occurs in which soda ash is not being used as dye activator (funny name, "dye activator", since it is actually the fabric that gets activated, not the dye). One dyer reported buying clearly labeled sodium carbonate from a swimming pool supplier or hardware store which was actually sodium BIcarbonate, a related chemical that does not produce an adequately high pH. This is very rare, but it has happened. Occasionally a dyer will confuse their bags of white chemicals and use urea when they mean to be using soda ash, which will produce very pale colors indeed. Urea does no harm but does nothing to fix the dye.
Also, fiber reactive dye can
go bad with time. Sometimes it lasts for only one year from the time your order
it from your supplier. The dye lasts for several years after manufacture, but
you have no idea how much time as elapsed before the manufacturer sold it to the
retailer, or before the retailer sold it to you. Storage conditions
matter, too. If the dye is in a hot car with the windows closed, in the sun, for
even just one day, or if it spends time in a hot delivery truck in the summer
that gets up to 120°F, the dye may spoil almost immediately.
can
go bad with time. Sometimes it lasts for only one year from the time your order
it from your supplier. The dye lasts for several years after manufacture, but
you have no idea how much time as elapsed before the manufacturer sold it to the
retailer, or before the retailer sold it to you. Storage conditions
matter, too. If the dye is in a hot car with the windows closed, in the sun, for
even just one day, or if it spends time in a hot delivery truck in the summer
that gets up to 120°F, the dye may spoil almost immediately.
As you can see, you have a number of possible factors to test. I would advise you to do some test batiks, running through all of these variables, but using just a few scrawls of hot wax on fabric, rather than any artful design. You should be able to narrow down just what your problems may be.
One last detail:
there is webbing where the wax has cracked leaving the color pale and almost bleached out

If you do not like the amount of crackle you are getting in your dyeing, use a higher proportion of beeswax, or use pure beeswax instead of beeswax mixed with paraffin. You may substitute synthetic microcrystalline or "sticky" wax for beeswax, for the sake of economy.
(Please help support this web site. Thank you.)
Message: Hi,
I am a batik "artist" and I am writing as you you have one of the most informative sites that I have been to. The question I have for you is about staining of the white or waxed areas with other colors and the other I have for you you is about dharma's Navy blue.
1. Have you had problems with the overdye staining the white areas and also the pre dyed areas of the fabric leaving the entire batik dull and and dirty looking? If so is there a way to avoid this aside from adding more synthrapol to the boiling and final washing (yes I rinse all the shirts that I do but this has become an issue as of late, leaving me with many "dirty" or "old looking batiks").
There are two ways in which a fiber reactive dye such as Procion MX dye can back-stain fabric.
One is by having still-reactive dye in the fabric when you go to rinse it out. When this
 happens, the unreacted dye can get on the fabric in the wrong place and stain it
permanently by reacting with it. There is no way to solve this problem after it
happens, since the dye in the wrong places is as firmly attached as that in the
right places. The solution is to prevent this by making sure that the dye that
you have applied has fully reacted before you wash it out. Some of the dye will
have reacted with the fabric and become attached to it, while some will have
reacted with the water ("hydrolyzed") and will just have gone bad. Hydrolyzed
dye is not a threat because it cannot react. The easy way to make sure that all
your dye has reacted is to give it plenty of time; for example, if your dye
studio is at least 70°F (21°C), leave your dye reaction to run
overnight, while making sure (with either urea in the dye mixture, or plastic
over the fabric) that the dye stays moist on the fabric throughout the entire
reaction time.
happens, the unreacted dye can get on the fabric in the wrong place and stain it
permanently by reacting with it. There is no way to solve this problem after it
happens, since the dye in the wrong places is as firmly attached as that in the
right places. The solution is to prevent this by making sure that the dye that
you have applied has fully reacted before you wash it out. Some of the dye will
have reacted with the fabric and become attached to it, while some will have
reacted with the water ("hydrolyzed") and will just have gone bad. Hydrolyzed
dye is not a threat because it cannot react. The easy way to make sure that all
your dye has reacted is to give it plenty of time; for example, if your dye
studio is at least 70°F (21°C), leave your dye reaction to run
overnight, while making sure (with either urea in the dye mixture, or plastic
over the fabric) that the dye stays moist on the fabric throughout the entire
reaction time.If your dye reaction is too cool, then it will not complete in the time alloted. You may need to take care to use warmer water, always, of course, remaining below the hot temperature at which the batik wax will soften. Water that is 90°F or 100°F (32°C or 38°C) will help speed the dye reaction without softening your wax. There are various ways to keep your dye reaction warm, but that is the subject of another article.
The other form of backstaining is caused by dye that has already hydrolyzed, so it cannot form the permanent covalent bond that holds Procion MX dye to the fabric under optimal conditions. However, the dye can still associate with the fabric, albeit more loosely, in much the same way that all-purpose dye associates with cotton. Once this has happened, the solution is HOT water. When you rinse out your dye after dyeing, you start with cool water to remove all dye auxiliaries such as soda ash or salt. After that, you should use hot water to remove the dye, at least 140°F (60°C) if possible, and soak the fabric in the hot water for a little while. Hotter water is more effective still at removing excess unattached dye. A fairly short washing in nearly-boiling water will remove excess unattached Procion MX dye very effectively. This should be happening already when you boil out your wax after you are done batiking, shouldn't it?
There can be added complications that make
 it
harder to wash out dye. If the water is hard, the hardness minerals can form
complexes with the dyes which are much more difficult to wash out. In this case,
add a small amount of sodium hexametaphosphate to the water for every step of
dyeing, even the dye wash-out stage if necessary. See my FAQ on "We have very hard water.
Should I use distilled or spring water instead of tap
water?".
it
harder to wash out dye. If the water is hard, the hardness minerals can form
complexes with the dyes which are much more difficult to wash out. In this case,
add a small amount of sodium hexametaphosphate to the water for every step of
dyeing, even the dye wash-out stage if necessary. See my FAQ on "We have very hard water.
Should I use distilled or spring water instead of tap
water?".Another complication that can make dye appear
 to
be difficult to wash out is the presence of sizing in the fabric. This should
not be a problem if you are starting with PFD ("prepared for dyeing") fabric. If
there is starch in the fabric, a drop of tincture of iodine, placed on the
apparently clean fabric, will reveal the presence of the starch by turning blue.
Starch is extraordinarily difficult to full remove from fabric. Even boiling
with soda ash will not do it. Starch in the fabric will react with the dye in
just the same way as the cellulose in the fabric, but will gradually wash out,
giving the appearance of poor washfastness. Never dye fabric that has been sized
with starch, and beware of other sizings as well, which may be equally
problematic for one reason or another.
to
be difficult to wash out is the presence of sizing in the fabric. This should
not be a problem if you are starting with PFD ("prepared for dyeing") fabric. If
there is starch in the fabric, a drop of tincture of iodine, placed on the
apparently clean fabric, will reveal the presence of the starch by turning blue.
Starch is extraordinarily difficult to full remove from fabric. Even boiling
with soda ash will not do it. Starch in the fabric will react with the dye in
just the same way as the cellulose in the fabric, but will gradually wash out,
giving the appearance of poor washfastness. Never dye fabric that has been sized
with starch, and beware of other sizings as well, which may be equally
problematic for one reason or another.A more common complication that leads to difficult wash-out of excess dye is failure to use a cool rinse before washing in hot water. The presence of auxiliary chemicals such as salt or soda ash, when you put the fabric in hot water, may encourage the dye to form those loose associations that are like those of the direct dye in all-purpose dye mixtures. These associations makes washing-out so much more difficult! Never do you very first rinse of Procion MX or other fiber reactive dye in hot water. Always use cool water first to remove all auxiliary chemicals.
The other problem that I have been having in the past 6 months is the navy blue from Dharma not being as deep as I would like to have it (I do many night batiks and this is a color that I use to set off the rest of the colors) when the boiling and washing is done I am finding that the color is washed out and there is webbing where the wax has cracked leaving the color pale and almost bleached out.
We have taken to filling buckets with water and letting it sit over night to eliminate any of the chlorine in the water but it seems like this may be happening in the the rinse or boiling time....please let me know if there is anything that you think may help.
Much thanks
When your dye is failing to properly react with your fabric, which is what you are describing here, there are a number of different possible reasons. Number one is dye studio temperature: at what temperature is your dye reaction taking place, and for how long are you leaving it at this temperature? Procion MX type dyes react with the fabric very poorly below about 70°F (21°C). The warmer the dye reaction, the faster it goes.
Another possible cause of a poor dye reaction is bad fabric. If the fabric is sized with who-knows-what modern polymer surface treatment, or treated to make it permanent-press or stain-resistant, there's no telling what will happen. Of course, fabric that is not 100% cotton (or silk or rayon) is not going to react well. Occasionally fabric is mistakenly sold under the label of "100% cotton" when it is actually 50% polyester, which will not take dye well. I would advise you to use only PFD fabric from trustworthy sources for your batik fabric. Any cotton or rayon fabric purchased from Dharma Trading Company, Jacquard Fabrics, or TestFabrics Inc., for example, should be fine. You just need to use PFD cotton or rayon fabric.

Sometimes a problem occurs in which soda ash is not being used as dye activator (funny name, "dye activator", since it is actually the fabric that gets activated, not the dye). One dyer reported buying clearly labeled sodium carbonate from a swimming pool supplier or hardware store which was actually sodium BIcarbonate, a related chemical that does not produce an adequately high pH. This is very rare, but it has happened. Occasionally a dyer will confuse their bags of white chemicals and use urea when they mean to be using soda ash, which will produce very pale colors indeed. Urea does no harm but does nothing to fix the dye.
Also, fiber reactive dye
 can
go bad with time. Sometimes it lasts for only one year from the time your order
it from your supplier. The dye lasts for several years after manufacture, but
you have no idea how much time as elapsed before the manufacturer sold it to the
retailer, or before the retailer sold it to you. Storage conditions
matter, too. If the dye is in a hot car with the windows closed, in the sun, for
even just one day, or if it spends time in a hot delivery truck in the summer
that gets up to 120°F, the dye may spoil almost immediately.
can
go bad with time. Sometimes it lasts for only one year from the time your order
it from your supplier. The dye lasts for several years after manufacture, but
you have no idea how much time as elapsed before the manufacturer sold it to the
retailer, or before the retailer sold it to you. Storage conditions
matter, too. If the dye is in a hot car with the windows closed, in the sun, for
even just one day, or if it spends time in a hot delivery truck in the summer
that gets up to 120°F, the dye may spoil almost immediately.As you can see, you have a number of possible factors to test. I would advise you to do some test batiks, running through all of these variables, but using just a few scrawls of hot wax on fabric, rather than any artful design. You should be able to narrow down just what your problems may be.
One last detail:
there is webbing where the wax has cracked leaving the color pale and almost bleached out

If you do not like the amount of crackle you are getting in your dyeing, use a higher proportion of beeswax, or use pure beeswax instead of beeswax mixed with paraffin. You may substitute synthetic microcrystalline or "sticky" wax for beeswax, for the sake of economy.
(Please help support this web site. Thank you.)
Wednesday, November 21, 2007
the chemical, biological and physical processes and properties that generate the colours in clothing
Name: Barry
Message: hi,
I have really enjoyed your site. I am an undergrad student in Toronto, Canada, and I'm in a science course, 'Understanding Colour'. I am wondering if you have some ideas for web and non-web resources for a project I am working on?
My topic is Colours in Clothing (dyes). We have been asked to research the chemical, biological and physical processes and properties that generate the colours. I have found many books in our science library but they require a more advanced understanding of organic chemistry than I have, and I would like to start off with a clear understanding of the major ideas and concepts. Do you have any suggestions?
I have read your lesson plan and I really enjoyed it. I need more sources which are as accessible as yours.
Unfortunately, I don't know of other equally accessible sources, printed or online; this is one of my major motivations in writing the articles on my web site. Books on dyeing in general either show the chemistry and are impenetrable to beginners, or ignore the chemical structures altogether. I want to make the chemistry of dyeing more accessible and interesting to people who don't already understand the language of the technical information that is available.
Here is the most important point to know about dyes: there are two different aspects to how a dye works. One is how it attaches to the fiber, and the other is how it makes the color. These two properties may be provided by entirely different parts of the dye molecule, though this is not always the case. A third property, solubility in water, is often provided by the presence of sulfonate groups on the dye molecule.
For example, in the case of reactive dyes, there is one section of the dye molecule that is called the chromophore; this provides the color of the dye. Each molecule of reactive dye also includes a section known as the reactive section; this is the part that actually reacts with the cellulose or protein fiber in order to form a permanent covalent bond.
The other common type of dye for cellulose fibers is called direct dye. These are large molecules that do not have a reactive section. The colored section of the molecule is all that there is. Its size helps it to loosely associate with the cellulose fiber, though higher temperatures tend to break these weak attractions and wash out the dye.
Protein fibers, such as silk and wool, are more complicated, since they are made of repeats of some twenty amino acids, instead of repeats of a single sugar subunit as in the case of cellulose.
For more information on how different types of dyes attach to clothing fibers, see my page, "What kinds of chemical bonds attach dyes to fibers? ".
The question of how different dye molecules make the different colors is much more complex and difficult to understand than the question of how dyes attach to clothing fibers. If you look at drawings of the structures of a number of different dyes, you will see that most of them contain multiple aromatic ring structures, or at least alternating single and double bonds. Certain arrangements of these cause a molecule to absorb specific wavelengths of light. When a molecule absorbs light in the visible region of the electromagnetic spectrum, the remaining wavelengths that are reflected from the substance give it its color. If red light is absorbed and other light reflected, you get the appearance of green. If blue is absorbed, you see orange; if violet is absorbed, you see yellow (which may be a mixture of red, orange, yellow, and green light); if yellow is absorbed, you see purple (which is a combination of red and blue wavelengths together.
A website called Stainsfile contains a worthwhile discussion about what makes different molecules have color, which you will want to read.
Specific colors tend to be created by similar structures among different classes of dye. For example, almost all turquoise blue dyes are based on a copper phthalocyanine ring, with various chemical groups attached to give the dye the ability to react with cotton, or act as a direct dye, or an acid dye for protein fibers, etc. You can see a picture of the structure for Color Index Reactive Blue 140, Procion turquoise MX-G, on the left; it is an enormous ring structure with a copper atom in the middle. This dye is actually a mixture of several closely related molecules, with either two or three reactive groups and one or two sulfonate groups attached at any of several points of the phthalocyanine ring.
Dr. Steve Mihok has a wonderful summation of different chromophores in all sorts of blue dyes on his site. Blue dyes can contain any of several different chromophores, including anthraquinones and triphenodioxazines. The triphenodioxazine structure contains five adjacent rings, including two nitrogens and two oxygens in the rings. Anthraquinone, the parent of the anthraquinone dyes, consists of a triple ring structure with two oxygen atoms attached to the middle ring. Indigo, the only widely-used good natural blue dye, though now mainly used in its synthetic form, gives its name to the indanthrone dyes. One way that purple can be produced is found in a close chemical relative of indigo, 6,6'dibromoindigo, which is the famous dye of antiquity known as Tyrian Purple.
Anthraquinone structures can also produce an orange or red color, depending on the attached substituent groups. The most common chromophores for red, orange, and yellow dyes are azo dye structures.
The best basic book I know of for beginners in this topic is
 Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.
Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.
(Please help support this web site. Thank you.)
Message: hi,
I have really enjoyed your site. I am an undergrad student in Toronto, Canada, and I'm in a science course, 'Understanding Colour'. I am wondering if you have some ideas for web and non-web resources for a project I am working on?
My topic is Colours in Clothing (dyes). We have been asked to research the chemical, biological and physical processes and properties that generate the colours. I have found many books in our science library but they require a more advanced understanding of organic chemistry than I have, and I would like to start off with a clear understanding of the major ideas and concepts. Do you have any suggestions?
I have read your lesson plan and I really enjoyed it. I need more sources which are as accessible as yours.
Unfortunately, I don't know of other equally accessible sources, printed or online; this is one of my major motivations in writing the articles on my web site. Books on dyeing in general either show the chemistry and are impenetrable to beginners, or ignore the chemical structures altogether. I want to make the chemistry of dyeing more accessible and interesting to people who don't already understand the language of the technical information that is available.
Reactions of various reactive dyes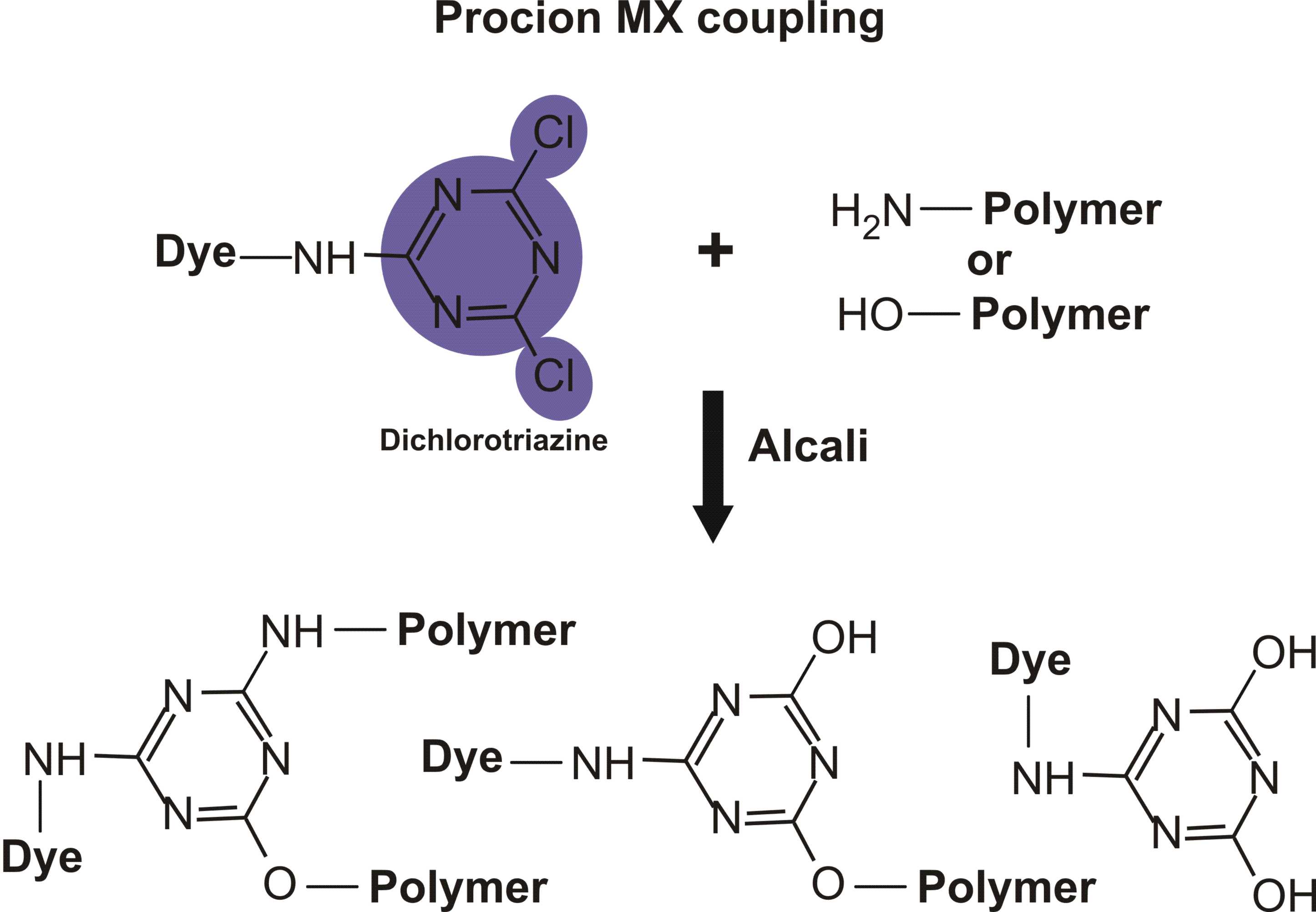
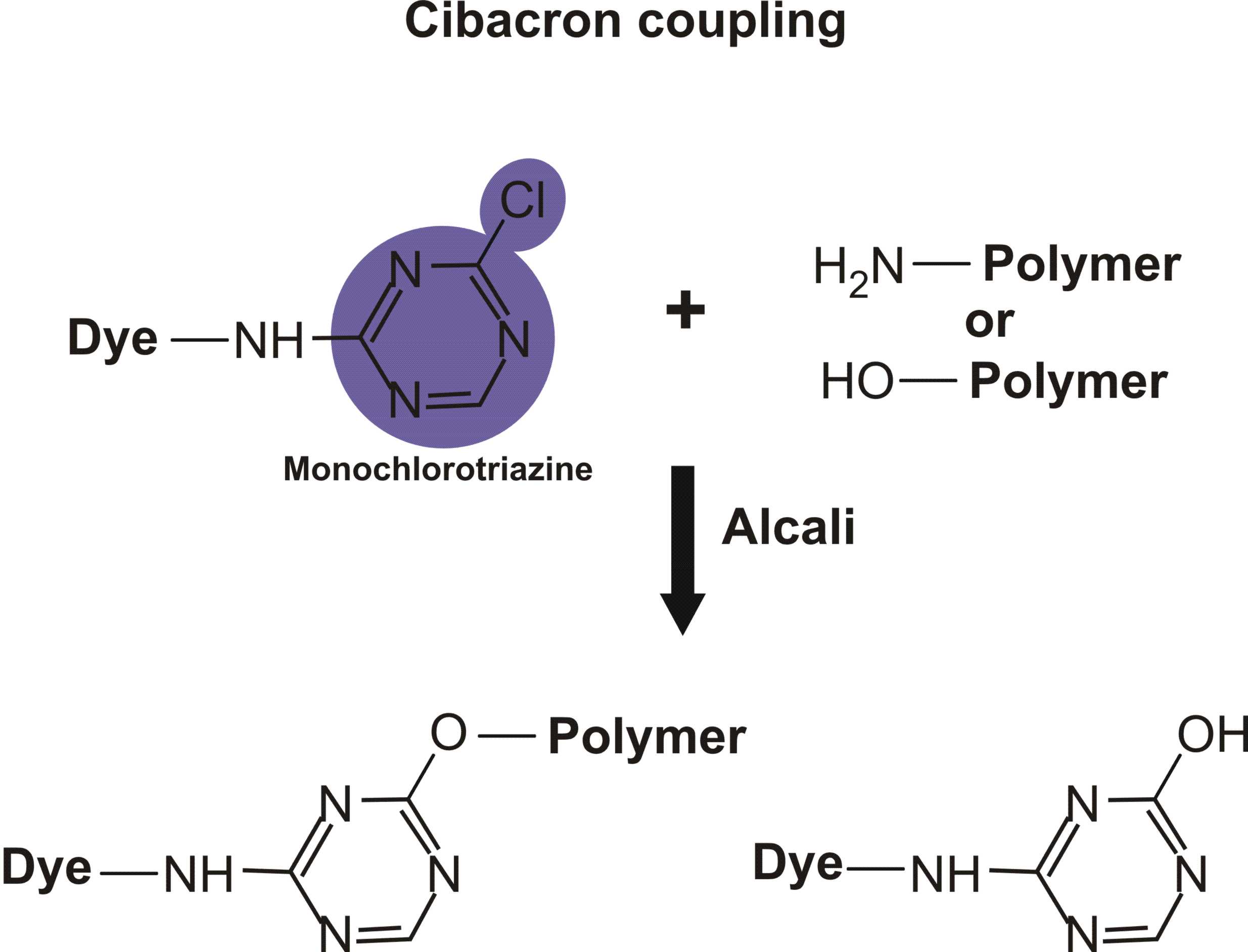
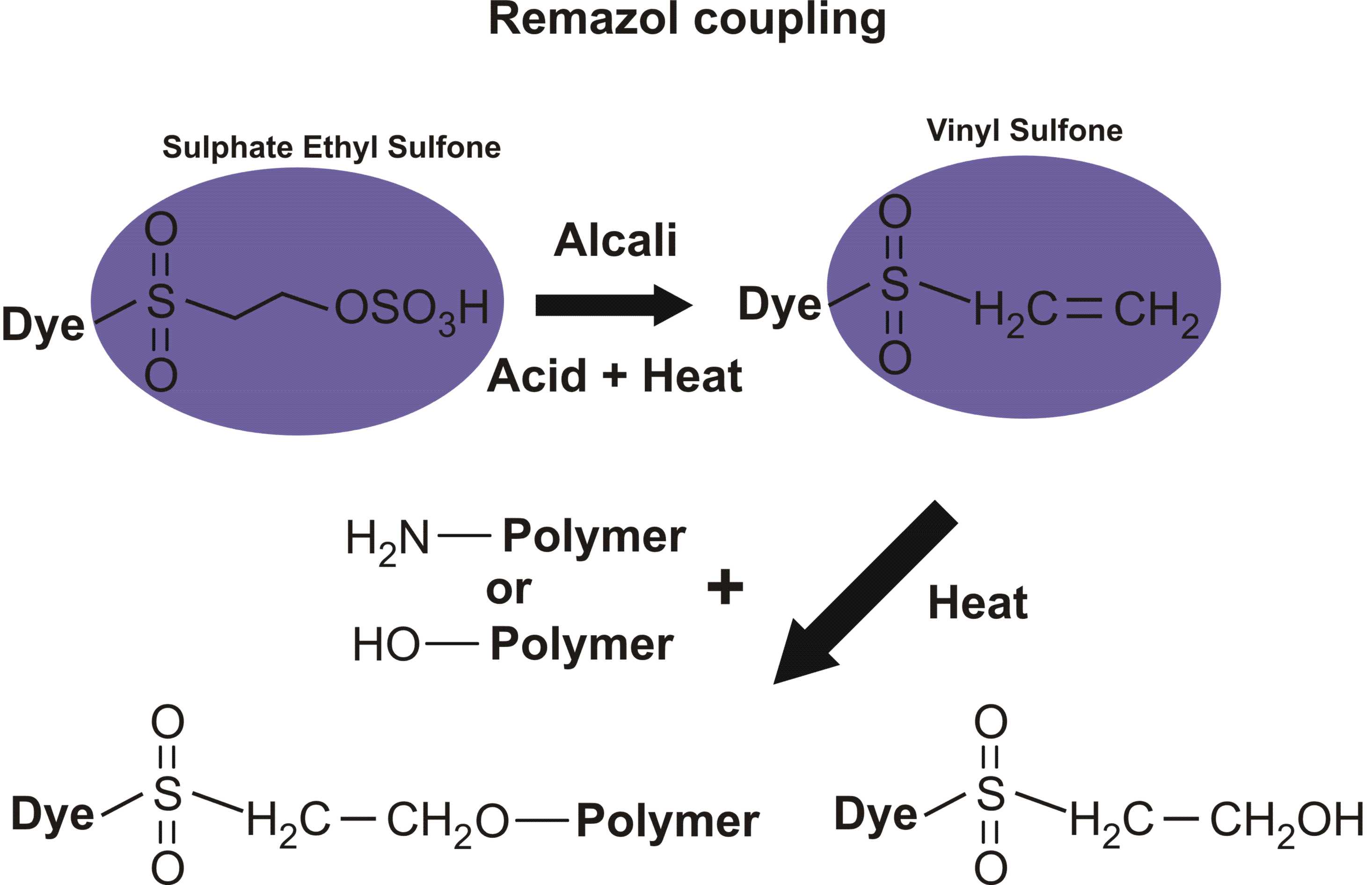



Structures of various reactive dyes
(chromophore is shown on the left side, reactive group on the right)
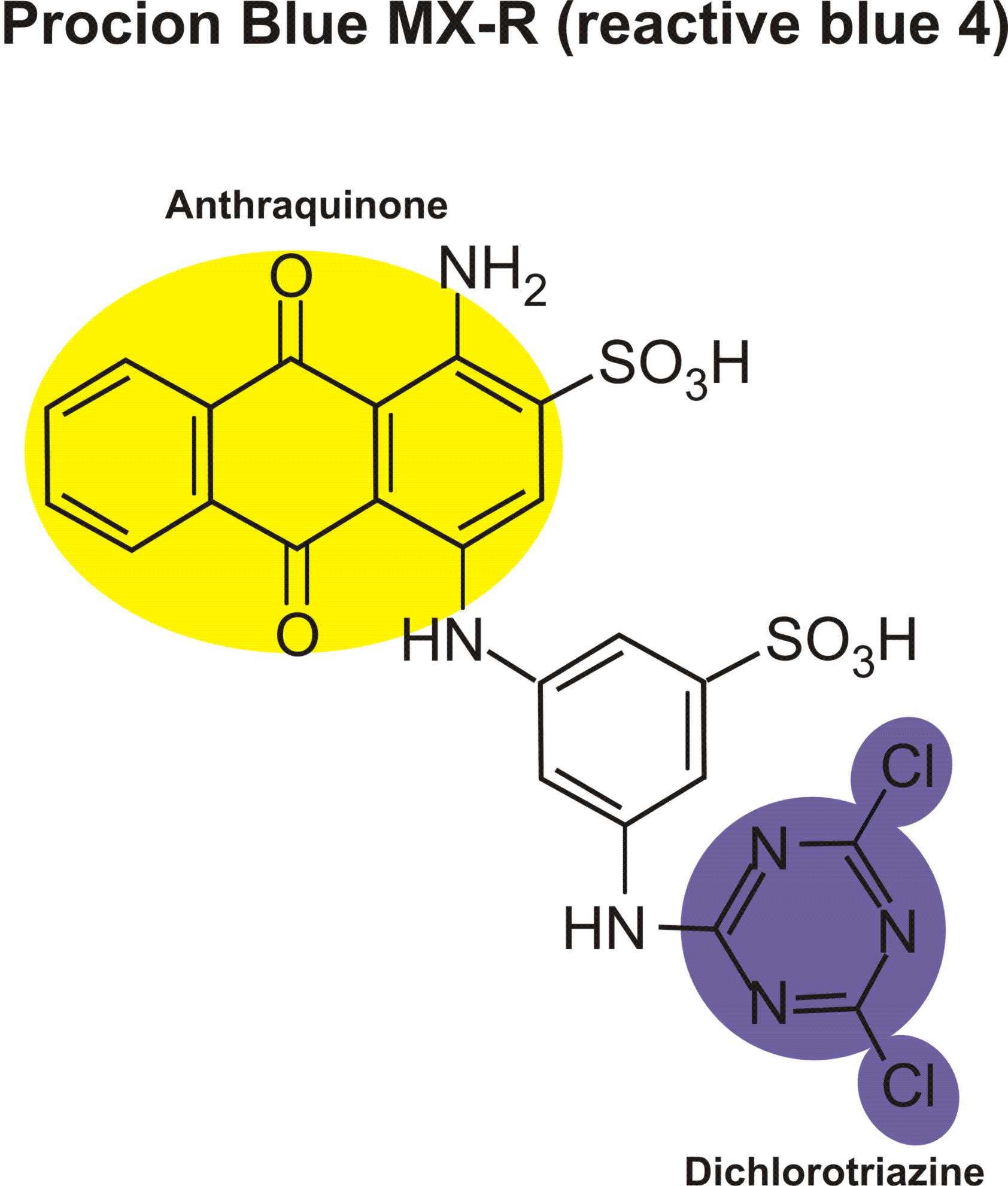
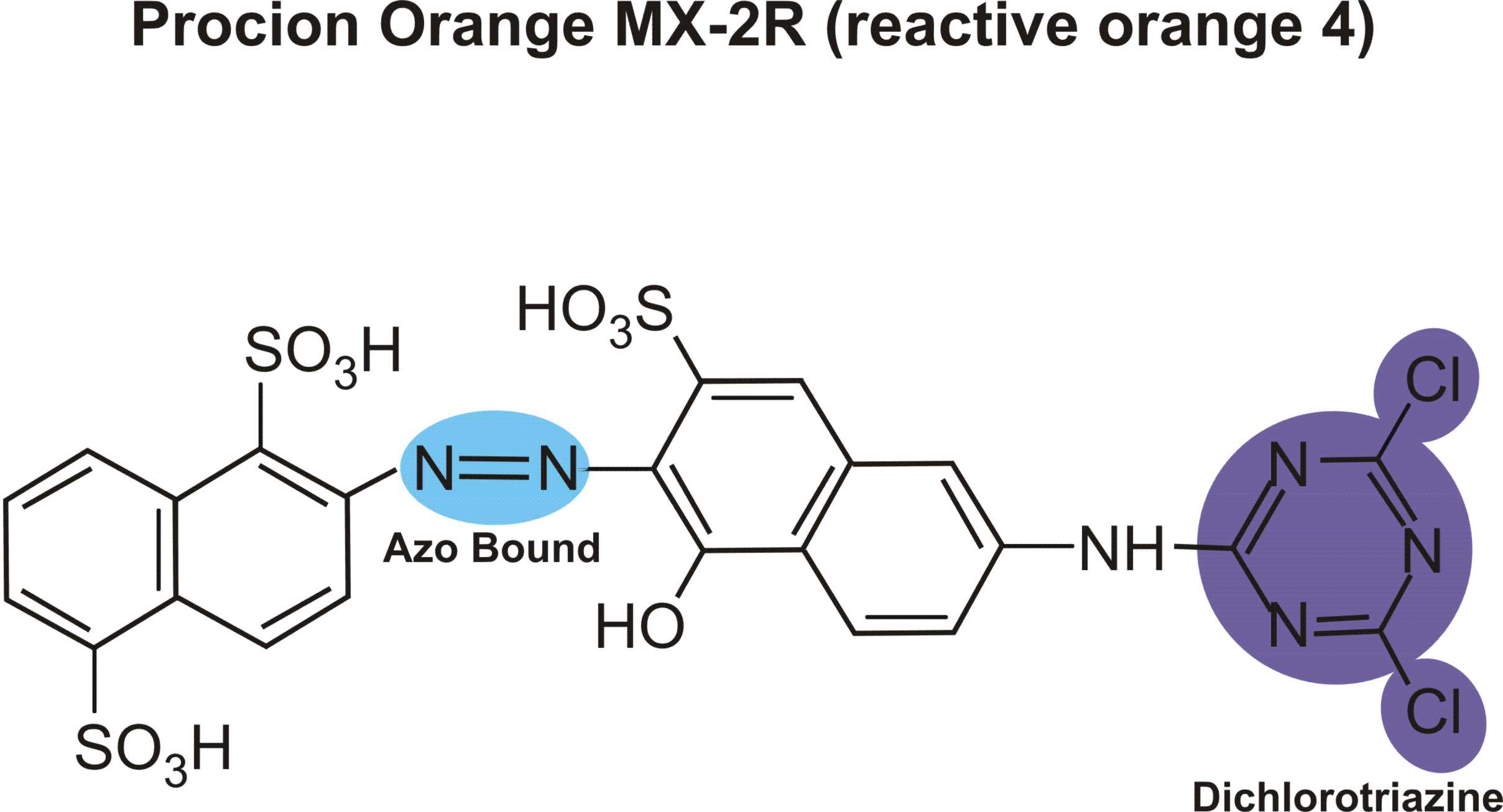
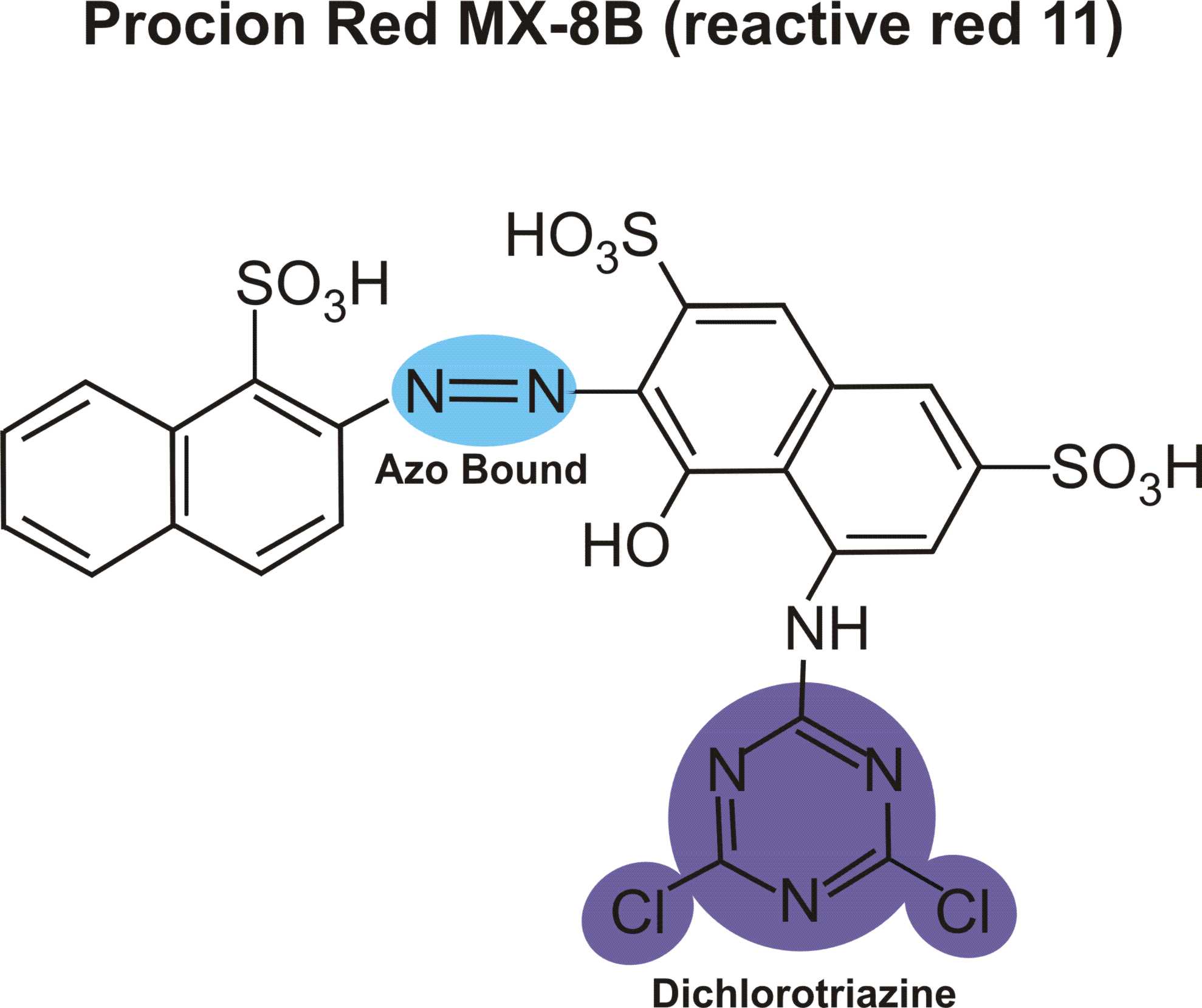
(The illustrations shown above, with the chromophore shown in yellow or blue and the reactive group shown in violet, were drawn by Dr. Francois Denis, who kindly gave permission for me to use them for educational purposes.)
(chromophore is shown on the left side, reactive group on the right)



(The illustrations shown above, with the chromophore shown in yellow or blue and the reactive group shown in violet, were drawn by Dr. Francois Denis, who kindly gave permission for me to use them for educational purposes.)
Here is the most important point to know about dyes: there are two different aspects to how a dye works. One is how it attaches to the fiber, and the other is how it makes the color. These two properties may be provided by entirely different parts of the dye molecule, though this is not always the case. A third property, solubility in water, is often provided by the presence of sulfonate groups on the dye molecule.
For example, in the case of reactive dyes, there is one section of the dye molecule that is called the chromophore; this provides the color of the dye. Each molecule of reactive dye also includes a section known as the reactive section; this is the part that actually reacts with the cellulose or protein fiber in order to form a permanent covalent bond.
The other common type of dye for cellulose fibers is called direct dye. These are large molecules that do not have a reactive section. The colored section of the molecule is all that there is. Its size helps it to loosely associate with the cellulose fiber, though higher temperatures tend to break these weak attractions and wash out the dye.
Protein fibers, such as silk and wool, are more complicated, since they are made of repeats of some twenty amino acids, instead of repeats of a single sugar subunit as in the case of cellulose.
For more information on how different types of dyes attach to clothing fibers, see my page, "What kinds of chemical bonds attach dyes to fibers? ".
The question of how different dye molecules make the different colors is much more complex and difficult to understand than the question of how dyes attach to clothing fibers. If you look at drawings of the structures of a number of different dyes, you will see that most of them contain multiple aromatic ring structures, or at least alternating single and double bonds. Certain arrangements of these cause a molecule to absorb specific wavelengths of light. When a molecule absorbs light in the visible region of the electromagnetic spectrum, the remaining wavelengths that are reflected from the substance give it its color. If red light is absorbed and other light reflected, you get the appearance of green. If blue is absorbed, you see orange; if violet is absorbed, you see yellow (which may be a mixture of red, orange, yellow, and green light); if yellow is absorbed, you see purple (which is a combination of red and blue wavelengths together.
A website called Stainsfile contains a worthwhile discussion about what makes different molecules have color, which you will want to read.
Specific colors tend to be created by similar structures among different classes of dye. For example, almost all turquoise blue dyes are based on a copper phthalocyanine ring, with various chemical groups attached to give the dye the ability to react with cotton, or act as a direct dye, or an acid dye for protein fibers, etc. You can see a picture of the structure for Color Index Reactive Blue 140, Procion turquoise MX-G, on the left; it is an enormous ring structure with a copper atom in the middle. This dye is actually a mixture of several closely related molecules, with either two or three reactive groups and one or two sulfonate groups attached at any of several points of the phthalocyanine ring.
Dr. Steve Mihok has a wonderful summation of different chromophores in all sorts of blue dyes on his site. Blue dyes can contain any of several different chromophores, including anthraquinones and triphenodioxazines. The triphenodioxazine structure contains five adjacent rings, including two nitrogens and two oxygens in the rings. Anthraquinone, the parent of the anthraquinone dyes, consists of a triple ring structure with two oxygen atoms attached to the middle ring. Indigo, the only widely-used good natural blue dye, though now mainly used in its synthetic form, gives its name to the indanthrone dyes. One way that purple can be produced is found in a close chemical relative of indigo, 6,6'dibromoindigo, which is the famous dye of antiquity known as Tyrian Purple.
Anthraquinone structures can also produce an orange or red color, depending on the attached substituent groups. The most common chromophores for red, orange, and yellow dyes are azo dye structures.
The best basic book I know of for beginners in this topic is

 Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.
Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.(Please help support this web site. Thank you.)
Tuesday, November 20, 2007
Can I save soda ash mixtures for other tie dying batches in the future? If so, how long can I save it for, and in what conditions?
Name: Elisheva
Message: Can I save soda ash mixtures for other tie dying batches in the future? If so, how long can I save it for, and in what conditions?

Yes, you can save soda ash solutions indefinitely. Soda ash never goes bad. Mold will not grow in it, and it does not degrade chemically to something else. You can reuse it for presoaking your clothes for tie-dyeing until there is no more left in the bucket.

If you store your soda ash mixture in an open bucket, you have to be aware of two possible problems. One is rain, if you leave it outdoors. If rain falls in the soda ash mixture, then obviously it will be weaker than you want, so you should cover it to prevent this from happening. (This will also protect animals from trying to take a drink.) The other issue is evaporation, which will concentrate your soda ash solution and make it too strong. You can draw a line on the outside of the bucket, showing the current level of soda ash, then add water back before using it again, but of course you have to draw a new line after each use.
Your choice
in storage containers does matter, a little.
choice
in storage containers does matter, a little.
 More
than one dyer has reported that storing soda ash mixtures in thin plastic
gallon-sized milk jugs is a big mistake. The plastic is so thin and weak that
eventually the soda ash will eat through, creating an unwelcome mess. The same
plastic that is used in milk bottles is okay if it is in the form of a thick,
solid bucket. There is no problem with the empty jugs sold by some dye
suppliers, nor with the plastic jugs that vinegar is sold in.
More
than one dyer has reported that storing soda ash mixtures in thin plastic
gallon-sized milk jugs is a big mistake. The plastic is so thin and weak that
eventually the soda ash will eat through, creating an unwelcome mess. The same
plastic that is used in milk bottles is okay if it is in the form of a thick,
solid bucket. There is no problem with the empty jugs sold by some dye
suppliers, nor with the plastic jugs that vinegar is sold in.
(Please help support this web site. Thank you.)
Message: Can I save soda ash mixtures for other tie dying batches in the future? If so, how long can I save it for, and in what conditions?

Yes, you can save soda ash solutions indefinitely. Soda ash never goes bad. Mold will not grow in it, and it does not degrade chemically to something else. You can reuse it for presoaking your clothes for tie-dyeing until there is no more left in the bucket.

If you store your soda ash mixture in an open bucket, you have to be aware of two possible problems. One is rain, if you leave it outdoors. If rain falls in the soda ash mixture, then obviously it will be weaker than you want, so you should cover it to prevent this from happening. (This will also protect animals from trying to take a drink.) The other issue is evaporation, which will concentrate your soda ash solution and make it too strong. You can draw a line on the outside of the bucket, showing the current level of soda ash, then add water back before using it again, but of course you have to draw a new line after each use.
Your
 choice
in storage containers does matter, a little.
choice
in storage containers does matter, a little.
 More
than one dyer has reported that storing soda ash mixtures in thin plastic
gallon-sized milk jugs is a big mistake. The plastic is so thin and weak that
eventually the soda ash will eat through, creating an unwelcome mess. The same
plastic that is used in milk bottles is okay if it is in the form of a thick,
solid bucket. There is no problem with the empty jugs sold by some dye
suppliers, nor with the plastic jugs that vinegar is sold in.
More
than one dyer has reported that storing soda ash mixtures in thin plastic
gallon-sized milk jugs is a big mistake. The plastic is so thin and weak that
eventually the soda ash will eat through, creating an unwelcome mess. The same
plastic that is used in milk bottles is okay if it is in the form of a thick,
solid bucket. There is no problem with the empty jugs sold by some dye
suppliers, nor with the plastic jugs that vinegar is sold in.(Please help support this web site. Thank you.)
Monday, November 19, 2007
I have about 10m of natural wool to dye black, I bought a dye and tried a small off cut it turned out brown. So, to dye a natural wool, cream in colour, black, where did I go wrong and, more importantly, what do you suggest?
Name: Diane
Message: Hi,
I have about 10m of natural wool to dye black, I bought a dye and tried a small off cut it turned out brown. So, to dye a natural wool, cream in colour, black, where did I go wrong and, more importantly, what do you suggest? I look forward to hearing from you.
The first rule in dyeing anything black, regardless of what dye you use, is to use MORE DYE. It takes at least two to four times as much dye to get black as you would use for any other color.
You will also need to use an acid, such as vinegar or citric acid, when dyeing wool, carefully following a good recipe for the particular dye that you have. The amount required depends on the type of dye you are using. The manufacturers for Tintex Hot Water dye in Australia suggests using 1 cup (250 ml) of white vinegar per 10 liters of water when dyeing wool, silk, or nylon. You must use heat when dyeing wool, bringing the dyebath to a simmer and holding it for half an hour to an hour, depending on the dye—ordinary washing machine temperatures will not do—and be sure to use a large volume of water in order to get smooth, even solid coverage with your dye.


The next question is, what kind of dye are you using? If you are using an all-purpose dye (such as Rit® or Tintex® all-purpose dyes), then what you've seen is what many people have reported already for this class of dye. You will probably get a reasonably good black color if you use four boxes of dye for every pound of wool, though. The acid dyes contained in these all-purpose dyes are said to be leveling acid dyes, which are good for leveling (getting a perfectly smooth even color) but bad for washfastness (wash separately by hand in cool water only, or dry clean). For more washfast results, or for more satisfactory results in general, I would suggest that you buy an acid dye that is specifically designed for wool and other protein fibers, rather than an all-purpose dye.
The best dye for getting a rich, dark black on wool is the Lanaset Jet Black, also sold under the names Telana or Sabraset. (See "About Lanaset Dyes" for more information about these dyes.) It is a 1:2 metal complex dye with high lightfastness, which, unlike other acid dyes, is washfast even at 140°F. Most acid dyes are washfast only up to 105°F. The Lanaset Jet Black is actually a mixture of two different black dyes, but it does not separate out in direct dye application; it acts more like a single-hue, unmixed dye. Lanaset dyes are expensive, almost as expensive as all-purpose dyes, but they are widely agreed to be worth it. You should use 25 grams of this black dye per pound of wool, according to ProChem's instructions.
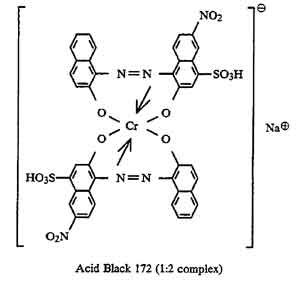
The black in PRO Chemical & Dye's Washfast Acid Dyes line is also a 1:2 metal complex dye; it is a single-hue, unmixed dye, Colour Index Acid Black 172, so it does not separate out in direct dye application. Acid Black 172 is actually one of the two dyes in the Lanaset Jet Black dye. It's much more economical than all-purpose dyes, as it is more concetrated. It is rated as 7 (out of 8) for lightfastness, which is good, and has been rated at a washfastness of 4-5 (out of 5) in warm water of 105°F. As
with the Jet Black Lanaset dye, you should use 25 grams of this black dye per
pound of wool. Using less dye per pound of wool will produce a brownish or
reddish color.
As
with the Jet Black Lanaset dye, you should use 25 grams of this black dye per
pound of wool. Using less dye per pound of wool will produce a brownish or
reddish color.
The black in Jacquard 639 Acid Black dye is a mixture; you are supposed to use up to three ounces (85 grams) of this dye per pound of wool in order to get a nice deep black, according to Jacquard Products' instructions.
(Please help support this web site. Thank you.)
Message: Hi,
I have about 10m of natural wool to dye black, I bought a dye and tried a small off cut it turned out brown. So, to dye a natural wool, cream in colour, black, where did I go wrong and, more importantly, what do you suggest? I look forward to hearing from you.
The first rule in dyeing anything black, regardless of what dye you use, is to use MORE DYE. It takes at least two to four times as much dye to get black as you would use for any other color.
You will also need to use an acid, such as vinegar or citric acid, when dyeing wool, carefully following a good recipe for the particular dye that you have. The amount required depends on the type of dye you are using. The manufacturers for Tintex Hot Water dye in Australia suggests using 1 cup (250 ml) of white vinegar per 10 liters of water when dyeing wool, silk, or nylon. You must use heat when dyeing wool, bringing the dyebath to a simmer and holding it for half an hour to an hour, depending on the dye—ordinary washing machine temperatures will not do—and be sure to use a large volume of water in order to get smooth, even solid coverage with your dye.


The next question is, what kind of dye are you using? If you are using an all-purpose dye (such as Rit® or Tintex® all-purpose dyes), then what you've seen is what many people have reported already for this class of dye. You will probably get a reasonably good black color if you use four boxes of dye for every pound of wool, though. The acid dyes contained in these all-purpose dyes are said to be leveling acid dyes, which are good for leveling (getting a perfectly smooth even color) but bad for washfastness (wash separately by hand in cool water only, or dry clean). For more washfast results, or for more satisfactory results in general, I would suggest that you buy an acid dye that is specifically designed for wool and other protein fibers, rather than an all-purpose dye.
The best dye for getting a rich, dark black on wool is the Lanaset Jet Black, also sold under the names Telana or Sabraset. (See "About Lanaset Dyes" for more information about these dyes.) It is a 1:2 metal complex dye with high lightfastness, which, unlike other acid dyes, is washfast even at 140°F. Most acid dyes are washfast only up to 105°F. The Lanaset Jet Black is actually a mixture of two different black dyes, but it does not separate out in direct dye application; it acts more like a single-hue, unmixed dye. Lanaset dyes are expensive, almost as expensive as all-purpose dyes, but they are widely agreed to be worth it. You should use 25 grams of this black dye per pound of wool, according to ProChem's instructions.

The black in PRO Chemical & Dye's Washfast Acid Dyes line is also a 1:2 metal complex dye; it is a single-hue, unmixed dye, Colour Index Acid Black 172, so it does not separate out in direct dye application. Acid Black 172 is actually one of the two dyes in the Lanaset Jet Black dye. It's much more economical than all-purpose dyes, as it is more concetrated. It is rated as 7 (out of 8) for lightfastness, which is good, and has been rated at a washfastness of 4-5 (out of 5) in warm water of 105°F.
 As
with the Jet Black Lanaset dye, you should use 25 grams of this black dye per
pound of wool. Using less dye per pound of wool will produce a brownish or
reddish color.
As
with the Jet Black Lanaset dye, you should use 25 grams of this black dye per
pound of wool. Using less dye per pound of wool will produce a brownish or
reddish color.The black in Jacquard 639 Acid Black dye is a mixture; you are supposed to use up to three ounces (85 grams) of this dye per pound of wool in order to get a nice deep black, according to Jacquard Products' instructions.
(Please help support this web site. Thank you.)
Sunday, November 18, 2007
dyeing a fishing vest so that it will not scare the fish
Name: Wendell
Message: Dear Paula,
I have a fishing vest that is light tan and has a 100% nylon shell. A stretchy mesh section made of 70% nylon and 30% spandex covers the shoulders. I just want to dye it a darker color -- either brown or a dark olive.
The reason for this color change is because trout can see light colors even at a distance and this causes them to take cover and hide. The vest manufacturers only offer light colors except for a few, and even those are not dark enough. I am an underwater videographer and my research has proven this out. Many anglers ask me how they can dye them (they are expensive.....from $50 to $140) and that is why I searched the internet and found your site. I hope it will not be a complicated operation.
There are two different options to consider here: acid dyes versus fabric paints.
You can dye nylon, but there's a problem here to be aware of. Nylon requires heat to dye well, but spandex is extremely heat sensitive. I don't know how permanent the dye will be if you stick to temperatures that are low enough not to endanger the set of the spandex, and yet I am afraid that heat about 105°F may cause the spandex to lose its shape. What do the care instructions say about washing? Do they say to use cool water only?
See my page on "How to Dye Spandex". Here also is a link to a discussion of the different dyes you can use to dye nylon, posted in this blog on November 9, 2007.

Typical instructions for dyeing nylon call for gradually heating the fabric in a large cooking pot of dye dissolved in water, with an acid such as vinegar to adjust the pH, slowly raising the temperature to 205°F (96°C), and then holding the dyebath at that temperature for half an hour to an hour, after which the dyebath is allowed to cool to room temperature, and then the dyed garment is washed out. For example, see ProChem's instructions for dyeing with their Washfast Acid line of dyes. To dye a nylon/spandex garment, you will need to use a much lower temperature to avoid damaging the spandex. The spandex will not melt, and in fact may itself be dyed at 140°F, but any shape that was set into your spandex fabric at the time of manufacture will be reset by high heat.
 There is a safer alternative, another way to darken your vest so that it will
not alert the trout. Instead of using dye, you can use fabric paint
or pigment dyeing. Fabric paints and pigment dyes use an acrylic binder, instead
of a chemical interaction between dye and fiber, to hold the tiny particles of
pigment onto the fabric. You cannot get a smooth, perfect, even shade throughout
the garment, but instead a somewhat mottled effect, such as you see in
commercially pigment-dyed clothing. However, it seems as though a mottled effect
might actually be superior for your purposes, as compared to a smooth solid
color.
There is a safer alternative, another way to darken your vest so that it will
not alert the trout. Instead of using dye, you can use fabric paint
or pigment dyeing. Fabric paints and pigment dyes use an acrylic binder, instead
of a chemical interaction between dye and fiber, to hold the tiny particles of
pigment onto the fabric. You cannot get a smooth, perfect, even shade throughout
the garment, but instead a somewhat mottled effect, such as you see in
commercially pigment-dyed clothing. However, it seems as though a mottled effect
might actually be superior for your purposes, as compared to a smooth solid
color.
Some fabric paints require heat-setting, but less extensive exposure to heat than you would give it in a dyebath; others can be set by adding a chemical catalyst to the fabric paint, and some do not require any heat-setting at all. For example, Dharma Pigment Dye is supposed to be set for a few minutes in a hot dryer. (Let the paint dry thoroughly before putting it in the dryer.) Dye-na-flow, a thin, flowable paint made by Jacquard Products, can be diluted with up to 25% water; an additive that can be purchased separately, Jacquard Airfix, makes heat-setting unnecessary.
None of
these suggestions will work if the fabric has been treated in any way to make it
stain-resistant or water-repellent. Even a permanent-press finish can cause
problems with both fabric dyes and fabric paints. Surface finishes will repel
both the dyebath and the fabric paint. Try sprinkling water on the garment: if
it beads up, you will not be able to change the color of the garment. If the
water soaks in, chances are good that you can do it, though not
certain.
of
these suggestions will work if the fabric has been treated in any way to make it
stain-resistant or water-repellent. Even a permanent-press finish can cause
problems with both fabric dyes and fabric paints. Surface finishes will repel
both the dyebath and the fabric paint. Try sprinkling water on the garment: if
it beads up, you will not be able to change the color of the garment. If the
water soaks in, chances are good that you can do it, though not
certain.
(Please help support this web site. Thank you.)
Message: Dear Paula,
I have a fishing vest that is light tan and has a 100% nylon shell. A stretchy mesh section made of 70% nylon and 30% spandex covers the shoulders. I just want to dye it a darker color -- either brown or a dark olive.
The reason for this color change is because trout can see light colors even at a distance and this causes them to take cover and hide. The vest manufacturers only offer light colors except for a few, and even those are not dark enough. I am an underwater videographer and my research has proven this out. Many anglers ask me how they can dye them (they are expensive.....from $50 to $140) and that is why I searched the internet and found your site. I hope it will not be a complicated operation.
There are two different options to consider here: acid dyes versus fabric paints.
You can dye nylon, but there's a problem here to be aware of. Nylon requires heat to dye well, but spandex is extremely heat sensitive. I don't know how permanent the dye will be if you stick to temperatures that are low enough not to endanger the set of the spandex, and yet I am afraid that heat about 105°F may cause the spandex to lose its shape. What do the care instructions say about washing? Do they say to use cool water only?
See my page on "How to Dye Spandex". Here also is a link to a discussion of the different dyes you can use to dye nylon, posted in this blog on November 9, 2007.

Typical instructions for dyeing nylon call for gradually heating the fabric in a large cooking pot of dye dissolved in water, with an acid such as vinegar to adjust the pH, slowly raising the temperature to 205°F (96°C), and then holding the dyebath at that temperature for half an hour to an hour, after which the dyebath is allowed to cool to room temperature, and then the dyed garment is washed out. For example, see ProChem's instructions for dyeing with their Washfast Acid line of dyes. To dye a nylon/spandex garment, you will need to use a much lower temperature to avoid damaging the spandex. The spandex will not melt, and in fact may itself be dyed at 140°F, but any shape that was set into your spandex fabric at the time of manufacture will be reset by high heat.
 There is a safer alternative, another way to darken your vest so that it will
not alert the trout. Instead of using dye, you can use fabric paint
or pigment dyeing. Fabric paints and pigment dyes use an acrylic binder, instead
of a chemical interaction between dye and fiber, to hold the tiny particles of
pigment onto the fabric. You cannot get a smooth, perfect, even shade throughout
the garment, but instead a somewhat mottled effect, such as you see in
commercially pigment-dyed clothing. However, it seems as though a mottled effect
might actually be superior for your purposes, as compared to a smooth solid
color.
There is a safer alternative, another way to darken your vest so that it will
not alert the trout. Instead of using dye, you can use fabric paint
or pigment dyeing. Fabric paints and pigment dyes use an acrylic binder, instead
of a chemical interaction between dye and fiber, to hold the tiny particles of
pigment onto the fabric. You cannot get a smooth, perfect, even shade throughout
the garment, but instead a somewhat mottled effect, such as you see in
commercially pigment-dyed clothing. However, it seems as though a mottled effect
might actually be superior for your purposes, as compared to a smooth solid
color.Some fabric paints require heat-setting, but less extensive exposure to heat than you would give it in a dyebath; others can be set by adding a chemical catalyst to the fabric paint, and some do not require any heat-setting at all. For example, Dharma Pigment Dye is supposed to be set for a few minutes in a hot dryer. (Let the paint dry thoroughly before putting it in the dryer.) Dye-na-flow, a thin, flowable paint made by Jacquard Products, can be diluted with up to 25% water; an additive that can be purchased separately, Jacquard Airfix, makes heat-setting unnecessary.
None
 of
these suggestions will work if the fabric has been treated in any way to make it
stain-resistant or water-repellent. Even a permanent-press finish can cause
problems with both fabric dyes and fabric paints. Surface finishes will repel
both the dyebath and the fabric paint. Try sprinkling water on the garment: if
it beads up, you will not be able to change the color of the garment. If the
water soaks in, chances are good that you can do it, though not
certain.
of
these suggestions will work if the fabric has been treated in any way to make it
stain-resistant or water-repellent. Even a permanent-press finish can cause
problems with both fabric dyes and fabric paints. Surface finishes will repel
both the dyebath and the fabric paint. Try sprinkling water on the garment: if
it beads up, you will not be able to change the color of the garment. If the
water soaks in, chances are good that you can do it, though not
certain.(Please help support this web site. Thank you.)
Saturday, November 17, 2007
I want to make a rainbow swirl tie dye with black "stripes" streaming out from the center. How do I get the stripes?
Name: Marg
Message: I want to make a rainbow swirl tie dye with black "stripes" streaming out from the center. How do I get the stripes?

The usual method for adding the black stripes is, after applying the colored fiber reactive dye in the spiral design, to dip the still-tied disk of fabric into a pan that has a very shallow layer of black fiber reactive dye. Alternatively, you can apply the black dye to just the surface of one side of the dyed fabric bundle, using a foam brush. It's a little hard for most people to picture how well this works, until they've tried it.
Note that black dye needs to be mixed at a higher concentration than most dye colors. Use at least twice as much dye powder when mixing your black dye as you do for your other colors.
The picture of the Nebula Spiral tie-dyed t-shirt to the right is from the True Tie Dye DVD, Tie Dye 101, which explains how to make this and many other basic tie-dye designs.
I'm assuming that you are using a good fiber reactive dye for this, such as Procion MX dye.The black stripe technique is not going to work well if you are using an all-purpose dye, such as Rit® or Tintex® brand dyes. Here's a link to a page on how to tie-dye with all-purpose dye. To add the black stripe on after that laborious (and somewhat dangerous, with respect to scalding) process would require brushing the dye on as above, but then wrapping the fabric, still very wet with dye, in plastic wrap, and steaming it for half an hour, as you might steam vegetables, to set the black dye, followed by rinsing and then the use of a cationic dye fixative such as Retayne to set the dye more permanently. The technique for doing this with fiber reactive dyes such as Procion MX dyes is so much simpler and easier!
(Please help support this web site. Thank you.)
Message: I want to make a rainbow swirl tie dye with black "stripes" streaming out from the center. How do I get the stripes?

The usual method for adding the black stripes is, after applying the colored fiber reactive dye in the spiral design, to dip the still-tied disk of fabric into a pan that has a very shallow layer of black fiber reactive dye. Alternatively, you can apply the black dye to just the surface of one side of the dyed fabric bundle, using a foam brush. It's a little hard for most people to picture how well this works, until they've tried it.
Note that black dye needs to be mixed at a higher concentration than most dye colors. Use at least twice as much dye powder when mixing your black dye as you do for your other colors.
The picture of the Nebula Spiral tie-dyed t-shirt to the right is from the True Tie Dye DVD, Tie Dye 101, which explains how to make this and many other basic tie-dye designs.
I'm assuming that you are using a good fiber reactive dye for this, such as Procion MX dye.The black stripe technique is not going to work well if you are using an all-purpose dye, such as Rit® or Tintex® brand dyes. Here's a link to a page on how to tie-dye with all-purpose dye. To add the black stripe on after that laborious (and somewhat dangerous, with respect to scalding) process would require brushing the dye on as above, but then wrapping the fabric, still very wet with dye, in plastic wrap, and steaming it for half an hour, as you might steam vegetables, to set the black dye, followed by rinsing and then the use of a cationic dye fixative such as Retayne to set the dye more permanently. The technique for doing this with fiber reactive dyes such as Procion MX dyes is so much simpler and easier!
(Please help support this web site. Thank you.)
Friday, November 16, 2007
My daughter painted a banner on a sheet with crayola washable paint. I would like to make it into a quilt backing/blanket. Is there any way to set the paint and make this blanket washable?
Name: Cindy
Message: My daughter painted a banner on a sheet with Crayola washable paint. I would like to make it into a quilt backing/blanket. Is there any way to set the paint and make this blanket washable? I already asked Crayola- they had no answer. Any help would be appreciated.
 Washable paint cannot be made wash-proof. It
contains a detergent in order to make and keep it water-soluble and easy to
clean up for children. As time passes, it will become less washable, so that
even after washing a stain will be left behind, but this is not nearly adequate
to what you want. Most of the design will wash out, and the results will look
terrible. Even if you don't wash it, it's bound to become dampened at some point
during use as a blanket, and then the paint will rub onto whatever it is next
to. (At least it should wash out well when that happens.)
Washable paint cannot be made wash-proof. It
contains a detergent in order to make and keep it water-soluble and easy to
clean up for children. As time passes, it will become less washable, so that
even after washing a stain will be left behind, but this is not nearly adequate
to what you want. Most of the design will wash out, and the results will look
terrible. Even if you don't wash it, it's bound to become dampened at some point
during use as a blanket, and then the paint will rub onto whatever it is next
to. (At least it should wash out well when that happens.)
What you need to do is change your idea of how to make this banner permanent. Do not attempt to make it a quilt; instead, frame it and use it on the wall.
You could
buy artists' wooden stretcher bars, such as we use to stretch canvas
for oil paintings, place your banner face-down on the floor, place the stretcher
bars (first assembled into a sturdy rectangle) on top, then pull the edges
of the fabric over the wooden bars and nail them to the back of the stretcher
bars using small tacks or a staple gun. Pull the fabric taut as you do this, so
it does not sag.
could
buy artists' wooden stretcher bars, such as we use to stretch canvas
for oil paintings, place your banner face-down on the floor, place the stretcher
bars (first assembled into a sturdy rectangle) on top, then pull the edges
of the fabric over the wooden bars and nail them to the back of the stretcher
bars using small tacks or a staple gun. Pull the fabric taut as you do this, so
it does not sag.
For a smaller design, you can have your local copy shop photocopy
it, or parts of it, onto photo transfer paper made for photocopiers. The copy
shop has heat presses that are ideal for putting these transfers onto fabric or
t-shirts. Alternatively, you can scan the design in on your computer and print
it on inkjet photo transfer paper and then use an iron
to transfer the image to fabric, t-shirts, mousepads, etc. If the painted sheet
is large, you can scan in just one section at a time and print it out, perhaps
reassembling the whole thing by ironing the transfer for all of the sections
onto a single piece of fabric.
photocopy
it, or parts of it, onto photo transfer paper made for photocopiers. The copy
shop has heat presses that are ideal for putting these transfers onto fabric or
t-shirts. Alternatively, you can scan the design in on your computer and print
it on inkjet photo transfer paper and then use an iron
to transfer the image to fabric, t-shirts, mousepads, etc. If the painted sheet
is large, you can scan in just one section at a time and print it out, perhaps
reassembling the whole thing by ironing the transfer for all of the sections
onto a single piece of fabric.
If none of this works for you, just take a nice photograph of the design to remember it by,

 and then buy your daughter some good fabric
paints for her next banner. Good brands include Jacquard Textile Color, Lumiere, Neopaque, Setacolor, Dye-Na-Flow, and ProFab Textile Paints. If you
don't care how the fabric feels, you can give her ordinary artists' acrylics
(Liquitex Soft Body is preferred), but for a
softer paint that feels okay on clothing or in a quilt, you must use a good
fabric paint. It is possible to turn artists' acrylics into an acceptable fabric
paint by adding Fabric Medium. Two good brands to look for are
Liquitex and Golden. Your local craft store probably does not have this, but you
can mail-order it from Dick Blick or another good art supply
store.
and then buy your daughter some good fabric
paints for her next banner. Good brands include Jacquard Textile Color, Lumiere, Neopaque, Setacolor, Dye-Na-Flow, and ProFab Textile Paints. If you
don't care how the fabric feels, you can give her ordinary artists' acrylics
(Liquitex Soft Body is preferred), but for a
softer paint that feels okay on clothing or in a quilt, you must use a good
fabric paint. It is possible to turn artists' acrylics into an acceptable fabric
paint by adding Fabric Medium. Two good brands to look for are
Liquitex and Golden. Your local craft store probably does not have this, but you
can mail-order it from Dick Blick or another good art supply
store.
(Please help support this web site. Thank you.)
Message: My daughter painted a banner on a sheet with Crayola washable paint. I would like to make it into a quilt backing/blanket. Is there any way to set the paint and make this blanket washable? I already asked Crayola- they had no answer. Any help would be appreciated.
 Washable paint cannot be made wash-proof. It
contains a detergent in order to make and keep it water-soluble and easy to
clean up for children. As time passes, it will become less washable, so that
even after washing a stain will be left behind, but this is not nearly adequate
to what you want. Most of the design will wash out, and the results will look
terrible. Even if you don't wash it, it's bound to become dampened at some point
during use as a blanket, and then the paint will rub onto whatever it is next
to. (At least it should wash out well when that happens.)
Washable paint cannot be made wash-proof. It
contains a detergent in order to make and keep it water-soluble and easy to
clean up for children. As time passes, it will become less washable, so that
even after washing a stain will be left behind, but this is not nearly adequate
to what you want. Most of the design will wash out, and the results will look
terrible. Even if you don't wash it, it's bound to become dampened at some point
during use as a blanket, and then the paint will rub onto whatever it is next
to. (At least it should wash out well when that happens.) What you need to do is change your idea of how to make this banner permanent. Do not attempt to make it a quilt; instead, frame it and use it on the wall.
You
 could
buy artists' wooden stretcher bars, such as we use to stretch canvas
for oil paintings, place your banner face-down on the floor, place the stretcher
bars (first assembled into a sturdy rectangle) on top, then pull the edges
of the fabric over the wooden bars and nail them to the back of the stretcher
bars using small tacks or a staple gun. Pull the fabric taut as you do this, so
it does not sag.
could
buy artists' wooden stretcher bars, such as we use to stretch canvas
for oil paintings, place your banner face-down on the floor, place the stretcher
bars (first assembled into a sturdy rectangle) on top, then pull the edges
of the fabric over the wooden bars and nail them to the back of the stretcher
bars using small tacks or a staple gun. Pull the fabric taut as you do this, so
it does not sag.For a smaller design, you can have your local copy shop
 photocopy
it, or parts of it, onto photo transfer paper made for photocopiers. The copy
shop has heat presses that are ideal for putting these transfers onto fabric or
t-shirts. Alternatively, you can scan the design in on your computer and print
it on inkjet photo transfer paper and then use an iron
to transfer the image to fabric, t-shirts, mousepads, etc. If the painted sheet
is large, you can scan in just one section at a time and print it out, perhaps
reassembling the whole thing by ironing the transfer for all of the sections
onto a single piece of fabric.
photocopy
it, or parts of it, onto photo transfer paper made for photocopiers. The copy
shop has heat presses that are ideal for putting these transfers onto fabric or
t-shirts. Alternatively, you can scan the design in on your computer and print
it on inkjet photo transfer paper and then use an iron
to transfer the image to fabric, t-shirts, mousepads, etc. If the painted sheet
is large, you can scan in just one section at a time and print it out, perhaps
reassembling the whole thing by ironing the transfer for all of the sections
onto a single piece of fabric.If none of this works for you, just take a nice photograph of the design to remember it by,


(Please help support this web site. Thank you.)
Thursday, November 15, 2007
Dyeing bamboo sheets with Procion Mx dyes: is bamboo fabric sturdy enough to use soda ash on?
Name: Britta
Message: My mom bought a set of bamboo sheets. I have not seen them yet, it does not matter to me whether the threads are natural or synthetic (dyed or white), but I do want the end color to set. I prefer to use procion mx dyes, and have been doing so successfully on cotton (and some silk) for years. I trust that these dyes will work with bamboo, as it is a natural fiber. But, I do not trust that the bamboo is as sturdy as cotton, and I am hesitant to use soda ash fixer. Do you have a different idea? Should I heat-set it or would soda ash be okay?
Heat setting is not very useful on cellulose fibers such
as bamboo. Most
bamboo fiber is regenerated cellulose, just like rayon. Rayon is easy to dye
with soda ash and fiber reactive
dyes, such as Procion
MX dyes. The soda ash is
required.
such
as bamboo. Most
bamboo fiber is regenerated cellulose, just like rayon. Rayon is easy to dye
with soda ash and fiber reactive
dyes, such as Procion
MX dyes. The soda ash is
required.
I recommend that you treat bamboo fabric very gently when it is wet, because it is probably like rayon, which is much weaker and more susceptible to damage when wet than it is when dry. What you have to worry about is physical abrasion, and tears, not the high pH of soda ash. I've had no trouble in using soda ash and Procion MX dyes to dye fragile rayon in my home washing machine, which has good delicate settings, but I have shredded rayon (just in a small section, but enough to ruin the dress) when washing it in another machine that did not have a reliable delicate setting. If your washing machine is not gentle enough, you will have to dye and wash by hand.
A different kind of bamboo fiber, which is prepared enzymatically, retaining the original fiber length, without digesting and regenerating the cellulose, is a strong fiber, stronger than rayon, but I don't believe I have heard of this form of bamboo being used in sheets. It's relatively rare. You had better assume that your bamboo sheets are as fragile as any rayon.
Are you aiming for a solid color, or
a more interesting multicolor effect? For a solid color, dyeing in the
washing machine is by far the easiest
method. Otherwise, low water
immersion (LWI) dyeing is the easiest. Low water immersion dyeing is
the least likely to damage the sheets, since it does not require constant
agitation or stirring of the wet fabric. Then you have only the washing itself
to be careful about. You can choose dramatic or very subtle effects, when using
the low water immersion technique, depending on what colors you choose, and how
much dye you use overall. A neutral pre-mixed color will separate out
wonderfully into surprising multi-colored effects, in LWI.
or
a more interesting multicolor effect? For a solid color, dyeing in the
washing machine is by far the easiest
method. Otherwise, low water
immersion (LWI) dyeing is the easiest. Low water immersion dyeing is
the least likely to damage the sheets, since it does not require constant
agitation or stirring of the wet fabric. Then you have only the washing itself
to be careful about. You can choose dramatic or very subtle effects, when using
the low water immersion technique, depending on what colors you choose, and how
much dye you use overall. A neutral pre-mixed color will separate out
wonderfully into surprising multi-colored effects, in LWI.
Note that the thread used to hem the edges of your bamboo sheets is likely to be made of polyester, which will not take the dye. This will probably not be a problem.
(Please help support this web site. Thank you.)
Message: My mom bought a set of bamboo sheets. I have not seen them yet, it does not matter to me whether the threads are natural or synthetic (dyed or white), but I do want the end color to set. I prefer to use procion mx dyes, and have been doing so successfully on cotton (and some silk) for years. I trust that these dyes will work with bamboo, as it is a natural fiber. But, I do not trust that the bamboo is as sturdy as cotton, and I am hesitant to use soda ash fixer. Do you have a different idea? Should I heat-set it or would soda ash be okay?
Heat setting is not very useful on cellulose fibers
 such
as bamboo. Most
bamboo fiber is regenerated cellulose, just like rayon. Rayon is easy to dye
with soda ash and fiber reactive
dyes, such as Procion
MX dyes. The soda ash is
required.
such
as bamboo. Most
bamboo fiber is regenerated cellulose, just like rayon. Rayon is easy to dye
with soda ash and fiber reactive
dyes, such as Procion
MX dyes. The soda ash is
required. I recommend that you treat bamboo fabric very gently when it is wet, because it is probably like rayon, which is much weaker and more susceptible to damage when wet than it is when dry. What you have to worry about is physical abrasion, and tears, not the high pH of soda ash. I've had no trouble in using soda ash and Procion MX dyes to dye fragile rayon in my home washing machine, which has good delicate settings, but I have shredded rayon (just in a small section, but enough to ruin the dress) when washing it in another machine that did not have a reliable delicate setting. If your washing machine is not gentle enough, you will have to dye and wash by hand.
A different kind of bamboo fiber, which is prepared enzymatically, retaining the original fiber length, without digesting and regenerating the cellulose, is a strong fiber, stronger than rayon, but I don't believe I have heard of this form of bamboo being used in sheets. It's relatively rare. You had better assume that your bamboo sheets are as fragile as any rayon.
Are you aiming for a solid color,
Note that the thread used to hem the edges of your bamboo sheets is likely to be made of polyester, which will not take the dye. This will probably not be a problem.
(Please help support this web site. Thank you.)
Wednesday, November 14, 2007
I have a light blue cotton shirt that doesn't come in black that I found on clearance. Now I want to make the shirt a medium gray color, don't care how even the color is, just don't want it baby blue colored.
Name: miles
Message: Hi there, I came across your site via a search for black cotton fabric dye. I have a light blue cotton shirt that doesn't come in black that I found on clearance. Now I want to make the shirt a medium gray color, don't care how even the color is, just don't want it baby blue colored. Can you tell me the best way to do this and sell me the necessary products? Thanks in advance.
If the shirt is not stain-resistant or permanent-press, and is 100% cotton, chances are good that you can change its color successfully. The stitching is probably polyester and will stay its current color. Will that be a problem for you? Sometimes, rarely, one panel of a shirt will turn out to be from a different bolt of fabric than another, and, although apparently the same color to begin with, will take the dye to a different intensity and no longer match the rest of the garment. So, there is never any guarantee that a particular garment will dye successfully, but it usually works.

I advise against the use of all-purpose dyes, such as Rit® or Tintex®, because they require very hot water to attach as well as they can, which will probably shrink your shirt; they work best on the stovetop, but devoting a large cooking pot to dyeing is expensive for someone who rarely dyes, because you should never reuse a dyepot for food; and because these dyes bleed in the laundry and fade quickly. (The problem of fading in the laundry can be solved with a cationic dye fixative such as Retayne, but that will increase lightfading, so it's not a perfect solution.)

The best dye to use would be a cool water fiber reactive dye, such as Procion MX dye. Don't buy a black dye when what you want is gray. Not all black dyes will make a grey when diluted; using a small amount of a black dye may produce a purplish color, or a greenish one, or a bluish one. I do not recommend attempting to mix your own dye color when attempting to make a grey, because a neutral grey is difficult to achieve, a project only for the expert dyer. If you want grey dye, buy it grey. A mixture that should work well for you is Jacquard's 230 Pearl Gray. Your pale blue will mix with the dye color, since dye is transparent, giving it a slightly blue tinge, but since the original color of your shirt is pale, it should not be a problem. Another option would be to buy Dylon Permanent Dye in "12 Black", if you can find this dye at your local fabric store; note that it is not the same dye as Dylon Cold Water dye (which works well but is a different dye) or Dylon Multi Purpose Dye (which you should avoid since it is another all-purpose dye).
The easiest way to dye anything a perfectly smooth solid color is
in the washing machine, but this can take a lot of dye, since there is so much
water involved. You can also do your dyeing in a five-gallon bucket, using a
long-handled plastic spoon or pole to stir constantly for about an hour, to make
sure that the dye is distributed evenly. I find this very tiring and prefer the
washing machine method. See "How can I dye
clothing or fabric in the washing machine?". The method is very
similar either way. In either case, for immersion dyeing, you will need
soda
ash, which raises the pH of
the cotton and allows the dye to react with it, and you will need some
ordinary salt to overcome
the high ratio of water to dye.
is
in the washing machine, but this can take a lot of dye, since there is so much
water involved. You can also do your dyeing in a five-gallon bucket, using a
long-handled plastic spoon or pole to stir constantly for about an hour, to make
sure that the dye is distributed evenly. I find this very tiring and prefer the
washing machine method. See "How can I dye
clothing or fabric in the washing machine?". The method is very
similar either way. In either case, for immersion dyeing, you will need
soda
ash, which raises the pH of
the cotton and allows the dye to react with it, and you will need some
ordinary salt to overcome
the high ratio of water to dye.
If you don't care at all how smooth and even the color is, then you can avoid all the stirring, and
the use of the washing machine, by using a different dyeing technique, called
low water immersion dyeing, or LWI. You use exactly the same materials, but put
them all in a small bucket, without stirring. This is an incredibly easy method
of dyeing. For more information, see "How to Do Low
Water Immersion Dyeing". Follow the instructions on that page. You
will need one to three teaspoons of dye (depending on how dark you want it), and
several teaspoons of soda ash; the use of salt is optional (but if you add it,
do so after dissolving the dye, since salt makes it more difficult to dissolve
dye). Note that a premixed dye color, such as grey, will separate out into
multiple colors on the fabric in LWI; this can be very rewarding, but if you
don't want color separation, you can use a single-hue, unmixed pure dye color,
as on my chart at this page: "Which Procion MX
colors are pure, and which mixtures?".
and
the use of the washing machine, by using a different dyeing technique, called
low water immersion dyeing, or LWI. You use exactly the same materials, but put
them all in a small bucket, without stirring. This is an incredibly easy method
of dyeing. For more information, see "How to Do Low
Water Immersion Dyeing". Follow the instructions on that page. You
will need one to three teaspoons of dye (depending on how dark you want it), and
several teaspoons of soda ash; the use of salt is optional (but if you add it,
do so after dissolving the dye, since salt makes it more difficult to dissolve
dye). Note that a premixed dye color, such as grey, will separate out into
multiple colors on the fabric in LWI; this can be very rewarding, but if you
don't want color separation, you can use a single-hue, unmixed pure dye color,
as on my chart at this page: "Which Procion MX
colors are pure, and which mixtures?".
For dyeing up to five pounds of fabric in the washing machine, you will need about 40 grams of dye, 7.5 pounds of salt, and one and a half cups of soda ash. For dyeing one pound of fabric in a five-gallon bucket, you will need about eight grams (or one tablespoon) of dye powder, 1.5 pounds of salt, and 5 tablespoons of soda ash. A small (2/3 ounce) jar of Jacquard Procion MX dye contains about 19 grams of dye powder. The small jar of dye is adequate for dyeing one or two shirts in a bucket, but you need to buy an eight-ounce jar of powder if you want to dye larger quantities of fabric in the washing machine.
Here are links to buy what you need through my web site, ordering from Amazon:
2/3 ounce jar Jacquard Procion MX 230 Pearl Gray ($3.14)
8 ounce jar Jacquard Procion MX 230 Pearl Gray ($9.61)
5 pound bag of soda ash dye activator ($4.76)
Depending on how you choose to do your dyeing, you may also need a
5-gallon plastic bucket, which you can buy locally, and several boxes of
non-iodized table salt or pickling salt from the grocery store.
(Please help support this web site. Thank you.)
Message: Hi there, I came across your site via a search for black cotton fabric dye. I have a light blue cotton shirt that doesn't come in black that I found on clearance. Now I want to make the shirt a medium gray color, don't care how even the color is, just don't want it baby blue colored. Can you tell me the best way to do this and sell me the necessary products? Thanks in advance.
If the shirt is not stain-resistant or permanent-press, and is 100% cotton, chances are good that you can change its color successfully. The stitching is probably polyester and will stay its current color. Will that be a problem for you? Sometimes, rarely, one panel of a shirt will turn out to be from a different bolt of fabric than another, and, although apparently the same color to begin with, will take the dye to a different intensity and no longer match the rest of the garment. So, there is never any guarantee that a particular garment will dye successfully, but it usually works.

I advise against the use of all-purpose dyes, such as Rit® or Tintex®, because they require very hot water to attach as well as they can, which will probably shrink your shirt; they work best on the stovetop, but devoting a large cooking pot to dyeing is expensive for someone who rarely dyes, because you should never reuse a dyepot for food; and because these dyes bleed in the laundry and fade quickly. (The problem of fading in the laundry can be solved with a cationic dye fixative such as Retayne, but that will increase lightfading, so it's not a perfect solution.)

The best dye to use would be a cool water fiber reactive dye, such as Procion MX dye. Don't buy a black dye when what you want is gray. Not all black dyes will make a grey when diluted; using a small amount of a black dye may produce a purplish color, or a greenish one, or a bluish one. I do not recommend attempting to mix your own dye color when attempting to make a grey, because a neutral grey is difficult to achieve, a project only for the expert dyer. If you want grey dye, buy it grey. A mixture that should work well for you is Jacquard's 230 Pearl Gray. Your pale blue will mix with the dye color, since dye is transparent, giving it a slightly blue tinge, but since the original color of your shirt is pale, it should not be a problem. Another option would be to buy Dylon Permanent Dye in "12 Black", if you can find this dye at your local fabric store; note that it is not the same dye as Dylon Cold Water dye (which works well but is a different dye) or Dylon Multi Purpose Dye (which you should avoid since it is another all-purpose dye).
The easiest way to dye anything a perfectly smooth solid color
 is
in the washing machine, but this can take a lot of dye, since there is so much
water involved. You can also do your dyeing in a five-gallon bucket, using a
long-handled plastic spoon or pole to stir constantly for about an hour, to make
sure that the dye is distributed evenly. I find this very tiring and prefer the
washing machine method. See "How can I dye
clothing or fabric in the washing machine?". The method is very
similar either way. In either case, for immersion dyeing, you will need
soda
ash, which raises the pH of
the cotton and allows the dye to react with it, and you will need some
ordinary salt to overcome
the high ratio of water to dye.
is
in the washing machine, but this can take a lot of dye, since there is so much
water involved. You can also do your dyeing in a five-gallon bucket, using a
long-handled plastic spoon or pole to stir constantly for about an hour, to make
sure that the dye is distributed evenly. I find this very tiring and prefer the
washing machine method. See "How can I dye
clothing or fabric in the washing machine?". The method is very
similar either way. In either case, for immersion dyeing, you will need
soda
ash, which raises the pH of
the cotton and allows the dye to react with it, and you will need some
ordinary salt to overcome
the high ratio of water to dye.If you don't care at all how smooth and even the color is, then you can avoid all the stirring,
 and
the use of the washing machine, by using a different dyeing technique, called
low water immersion dyeing, or LWI. You use exactly the same materials, but put
them all in a small bucket, without stirring. This is an incredibly easy method
of dyeing. For more information, see "How to Do Low
Water Immersion Dyeing". Follow the instructions on that page. You
will need one to three teaspoons of dye (depending on how dark you want it), and
several teaspoons of soda ash; the use of salt is optional (but if you add it,
do so after dissolving the dye, since salt makes it more difficult to dissolve
dye). Note that a premixed dye color, such as grey, will separate out into
multiple colors on the fabric in LWI; this can be very rewarding, but if you
don't want color separation, you can use a single-hue, unmixed pure dye color,
as on my chart at this page: "Which Procion MX
colors are pure, and which mixtures?".
and
the use of the washing machine, by using a different dyeing technique, called
low water immersion dyeing, or LWI. You use exactly the same materials, but put
them all in a small bucket, without stirring. This is an incredibly easy method
of dyeing. For more information, see "How to Do Low
Water Immersion Dyeing". Follow the instructions on that page. You
will need one to three teaspoons of dye (depending on how dark you want it), and
several teaspoons of soda ash; the use of salt is optional (but if you add it,
do so after dissolving the dye, since salt makes it more difficult to dissolve
dye). Note that a premixed dye color, such as grey, will separate out into
multiple colors on the fabric in LWI; this can be very rewarding, but if you
don't want color separation, you can use a single-hue, unmixed pure dye color,
as on my chart at this page: "Which Procion MX
colors are pure, and which mixtures?".For dyeing up to five pounds of fabric in the washing machine, you will need about 40 grams of dye, 7.5 pounds of salt, and one and a half cups of soda ash. For dyeing one pound of fabric in a five-gallon bucket, you will need about eight grams (or one tablespoon) of dye powder, 1.5 pounds of salt, and 5 tablespoons of soda ash. A small (2/3 ounce) jar of Jacquard Procion MX dye contains about 19 grams of dye powder. The small jar of dye is adequate for dyeing one or two shirts in a bucket, but you need to buy an eight-ounce jar of powder if you want to dye larger quantities of fabric in the washing machine.
Here are links to buy what you need through my web site, ordering from Amazon:
2/3 ounce jar Jacquard Procion MX 230 Pearl Gray ($3.14)
8 ounce jar Jacquard Procion MX 230 Pearl Gray ($9.61)
5 pound bag of soda ash dye activator ($4.76)
Depending on how you choose to do your dyeing, you may also need a
5-gallon plastic bucket, which you can buy locally, and several boxes of
non-iodized table salt or pickling salt from the grocery store.
(Please help support this web site. Thank you.)
Tuesday, November 13, 2007
I would need some azo dye structure of scarlet red, navilene brown, brilliant violet, remazol navy blue, remazol black, would you send through the mail
Name: sathish kumar
Message: Respected sir,
I am doing project related azo dyes, since I would need some azo dye structure of scarlet red, navilene brown, brilliant violet, remazol navy blue, remazol black, would you send through the mail
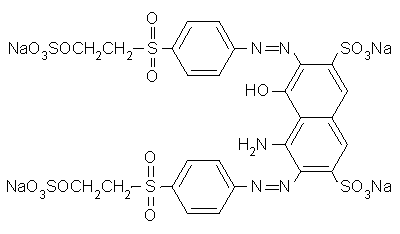
I have found a number of structures of Remazol dyes and have placed them in the chart near the bottom of the page here:
Vinyl Sulfone Fiber Reactive Dyes
Not all of the dyes whose structures you want are listed there, unfortunately. You may be able to find them by browsing through the Colour Index, published by the Society of Dyers and Colourists; contact your local universities and other institutions to see whether they subscribe to this publication.
I would particularly like to know the structure for Reactive Violet 5, a beautiful violet dye in the Remazol range. If you are able to find this, please forward it to me.
(Please help support this web site. Thank you.)
Message: Respected sir,
I am doing project related azo dyes, since I would need some azo dye structure of scarlet red, navilene brown, brilliant violet, remazol navy blue, remazol black, would you send through the mail

I have found a number of structures of Remazol dyes and have placed them in the chart near the bottom of the page here:
Vinyl Sulfone Fiber Reactive Dyes
Not all of the dyes whose structures you want are listed there, unfortunately. You may be able to find them by browsing through the Colour Index, published by the Society of Dyers and Colourists; contact your local universities and other institutions to see whether they subscribe to this publication.
I would particularly like to know the structure for Reactive Violet 5, a beautiful violet dye in the Remazol range. If you are able to find this, please forward it to me.
(Please help support this web site. Thank you.)
a science fair project on artificial dyes versus natural dyes
Name: Kegan A.
Message: Hello I am doing a science fair project on artificial dyes vs. natural dyes. I need one source from a person. My question is : do you know of any natural dyes that are from plants easily obtained? Im looking for red blue and yellow. Thank you in advance.
The majority of natural dyes are inferior to the better types of synthetic dyes in almost every characteristic: they are more difficult to apply correctly, they usually produce duller colors, and they tend to wash out much more easily in the laundry. They are frequently neither safer or kinder to the environment, since they are often used with dangerous heavy metals as mordants, and some natural dyes are poisonous. However, it is fun to use natural dyes, knowing where they come from and about their history. The challenge of getting a good result with more difficult materials is satisfying to many experienced dyers. There are two mordants, alum and tannic acid, which are quite safe to use, with only a reasonable amount of care. If you find a reliable recipe, you can succeed with natural dyeing, though it is more difficult than dyeing with a fiber reactive dye such as Procion MX dye.
When you dye your test samples for your project, you will get the best results by dyeing wool or silk. Do not try to dye polyester or acrylic, unless your experiment involves comparing it to natural fibers. Cotton is more difficult to dye than wool, but can be dyed with the dyes I will now describe to you.

The only good blue natural dye is indigo, which is produced by some fifty different plants around the world, none of which will be easily obtained by you. Indigo is a natural vat dye and is far too difficult to use for someone who has never dyed anything before. The chemicals required must be used only under adult supervision. Most indigo available for sale is synthetic and thus not suitable for your thesis, though the synthetic indigo is molecularly identical to indigo obtained from plants such as woad, or from the plant that is called indigo. Indigo does not require the use of mordants, but it requires a dangerously high pH, produced by dissolving lye (sodium hydroxide or NAOH) in water, as well as some other chemicals which must be used only with competent adult supervision.

Yellow is a relatively easy color to find among natural dyes. The easiest for you to find, and use, would be turmeric, which is a spice used in cooking. You can easily find a jar of powdered turmeric root at the grocery store. Turmeric is a natural direct dye and can be used even on cotton with no mordant at all. Turmeric will work on any natural fiber. The biggest drawback with turmeric is that it fades over time. It is susceptible to damage by light. If you dye a garment yellow with turmeric, you will need to dye it again every year. Like all natural dyes, turmeric works best when applied in a cooking pot on the stovetop. Add your turmeric to water, simmer it for an hour ("simmering" is cooking at a temperature just below boiling), then strain it through a coffee filter to remove bits of powder, then add your fabric to the dye water and simmer it for half an hour or longer.
Red natural dyes are much harder to find than yellows, but much easier to work with than blues. You can mail-order cochineal from a natural dyes supplier such as Aurora Silk or Earth Hues. Both of these companies are listed, with contact information, on my page of Sources for Dyeing Supplies Around the World. Cochineal is a wonderful red dye that will work on any natural fiber. It is more expensive than similar synthetic dyes, but works very well. If you are going to use it on cotton, you must also use mordants, such as alum AND tannic acid; order your mordants from the same company you order your cochineal from. Cochineal is found in the food color carmine, which is widely used to color foods such as Yoplait strawberry yogurt, and cosmetics such as lipsticks; it is made from the dried bodies of small insects that infest a certain kind of cactus.

For more information about dyeing with natural dyes, see About Natural Dyes. I recommend that you get a good book on natural dyeing. You will need a good recipe for applying your mordants to your fabric, before you apply your dye, except of course for turmeric as indicated above. The book "The Dyer's Companion ", by Dagmar Klos, contains good recipes for mordanting. Our public library has it; check to see if yours does, too. Look for other good books on natural dyes that you can use as sources for your recipes and for use in your bibliography.
(Please help support this web site. Thank you.)
Message: Hello I am doing a science fair project on artificial dyes vs. natural dyes. I need one source from a person. My question is : do you know of any natural dyes that are from plants easily obtained? Im looking for red blue and yellow. Thank you in advance.
The majority of natural dyes are inferior to the better types of synthetic dyes in almost every characteristic: they are more difficult to apply correctly, they usually produce duller colors, and they tend to wash out much more easily in the laundry. They are frequently neither safer or kinder to the environment, since they are often used with dangerous heavy metals as mordants, and some natural dyes are poisonous. However, it is fun to use natural dyes, knowing where they come from and about their history. The challenge of getting a good result with more difficult materials is satisfying to many experienced dyers. There are two mordants, alum and tannic acid, which are quite safe to use, with only a reasonable amount of care. If you find a reliable recipe, you can succeed with natural dyeing, though it is more difficult than dyeing with a fiber reactive dye such as Procion MX dye.
When you dye your test samples for your project, you will get the best results by dyeing wool or silk. Do not try to dye polyester or acrylic, unless your experiment involves comparing it to natural fibers. Cotton is more difficult to dye than wool, but can be dyed with the dyes I will now describe to you.

The only good blue natural dye is indigo, which is produced by some fifty different plants around the world, none of which will be easily obtained by you. Indigo is a natural vat dye and is far too difficult to use for someone who has never dyed anything before. The chemicals required must be used only under adult supervision. Most indigo available for sale is synthetic and thus not suitable for your thesis, though the synthetic indigo is molecularly identical to indigo obtained from plants such as woad, or from the plant that is called indigo. Indigo does not require the use of mordants, but it requires a dangerously high pH, produced by dissolving lye (sodium hydroxide or NAOH) in water, as well as some other chemicals which must be used only with competent adult supervision.

Yellow is a relatively easy color to find among natural dyes. The easiest for you to find, and use, would be turmeric, which is a spice used in cooking. You can easily find a jar of powdered turmeric root at the grocery store. Turmeric is a natural direct dye and can be used even on cotton with no mordant at all. Turmeric will work on any natural fiber. The biggest drawback with turmeric is that it fades over time. It is susceptible to damage by light. If you dye a garment yellow with turmeric, you will need to dye it again every year. Like all natural dyes, turmeric works best when applied in a cooking pot on the stovetop. Add your turmeric to water, simmer it for an hour ("simmering" is cooking at a temperature just below boiling), then strain it through a coffee filter to remove bits of powder, then add your fabric to the dye water and simmer it for half an hour or longer.
Red natural dyes are much harder to find than yellows, but much easier to work with than blues. You can mail-order cochineal from a natural dyes supplier such as Aurora Silk or Earth Hues. Both of these companies are listed, with contact information, on my page of Sources for Dyeing Supplies Around the World. Cochineal is a wonderful red dye that will work on any natural fiber. It is more expensive than similar synthetic dyes, but works very well. If you are going to use it on cotton, you must also use mordants, such as alum AND tannic acid; order your mordants from the same company you order your cochineal from. Cochineal is found in the food color carmine, which is widely used to color foods such as Yoplait strawberry yogurt, and cosmetics such as lipsticks; it is made from the dried bodies of small insects that infest a certain kind of cactus.

For more information about dyeing with natural dyes, see About Natural Dyes. I recommend that you get a good book on natural dyeing. You will need a good recipe for applying your mordants to your fabric, before you apply your dye, except of course for turmeric as indicated above. The book "The Dyer's Companion ", by Dagmar Klos, contains good recipes for mordanting. Our public library has it; check to see if yours does, too. Look for other good books on natural dyes that you can use as sources for your recipes and for use in your bibliography.
(Please help support this web site. Thank you.)
Monday, November 12, 2007
I am trying to find a dye house to dye large quantities of baby onesies for wholesale. Do you have any resources...
Name: Laura
Message: I am trying to find a dye house to dye large quantities of baby onesies for wholesale. Do you have any resources... Preferably in the NorthWest near Seattle. Thanks, Laura
I recommend that you try this page: Where can I find someone to dye my clothing for me?.
The top two entries are for solid-color garment redyeing, but the entries below that are hand dyers who have expressed an interest in doing custom orders such as yours.
(Please help support this web site. Thank you.)
Message: I am trying to find a dye house to dye large quantities of baby onesies for wholesale. Do you have any resources... Preferably in the NorthWest near Seattle. Thanks, Laura
I recommend that you try this page: Where can I find someone to dye my clothing for me?.
The top two entries are for solid-color garment redyeing, but the entries below that are hand dyers who have expressed an interest in doing custom orders such as yours.
(Please help support this web site. Thank you.)
Sunday, November 11, 2007
Do you make scrub sets? I have been going crazy trying to find someone that does sets and not just tops.
Name: Jennifer
Message: Hi,
Do you make scrub sets? I have been going crazy trying to find someone that does sets and not just tops. If you do, how much are they?
I am not able to do dyeing commercially these days, but you can certainly find a tie-dye artist to dye them for you, if you acquire some new 100% cotton scrubs that don't have any nasty permanent-press or stain-resistant finishes.
Dharma Trading Company sells two models of undyed 100% cotton scrubs for dyeing:
Plain Hospital Scrubs and Hospital Scrubs With Pockets.
To find a tie-dyer who does custom work, see this page:
"Where can I find someone to dye my clothing for me? =".
The top two entries are for solid-color garment redying, but the entries below that all cover tie-dyeing. Contact the person listed, check out their web site, make sure that they either will dye the clothing you send to them or will order scrubs to dye for you, and go on from there.
Some of those custom hand dyers will happily outfit your entire group with matching tie-dyed scrubs.
(Please help support this web site. Thank you.)
Message: Hi,
Do you make scrub sets? I have been going crazy trying to find someone that does sets and not just tops. If you do, how much are they?
I am not able to do dyeing commercially these days, but you can certainly find a tie-dye artist to dye them for you, if you acquire some new 100% cotton scrubs that don't have any nasty permanent-press or stain-resistant finishes.
Dharma Trading Company sells two models of undyed 100% cotton scrubs for dyeing:
Plain Hospital Scrubs and Hospital Scrubs With Pockets.
To find a tie-dyer who does custom work, see this page:
"Where can I find someone to dye my clothing for me? =".
The top two entries are for solid-color garment redying, but the entries below that all cover tie-dyeing. Contact the person listed, check out their web site, make sure that they either will dye the clothing you send to them or will order scrubs to dye for you, and go on from there.
Some of those custom hand dyers will happily outfit your entire group with matching tie-dyed scrubs.
(Please help support this web site. Thank you.)
Saturday, November 10, 2007
Will I have problems if I try to mix all purpose dye with fiber reactive dye to make a specific color? I was unable to find Rit Kelly Green color, so I bought some green Dylon Permanent Fabric Dye.
Name: Troy
Message: Will I have problems if I try to mix all purpose dye with fiber reactive dye to make a specific color? I was unable to find Rit Kelly Green color, so I bought some green Dylon Permanent Fabric Dye. I am going to mix it with some blue Rit dye that I already had. Will this end up working out?


It's definitely not a good idea to dye with fiber reactive dye (the Dylon® Permanent dye) and all-purpose dye (the Rit® dye) in the same step. Their requirements for attaching to the fabric are too different. The best way to combine these two dyes is to first dye with one, wash out the excess dye and chemicals, then dye with the other as an entirely different step. The Dylon Permanent dye is chemically set with a high pH, already included in the mix, whereas the Rit All Purpose dye requires high heat to set as well as it can, though it never sets very permanently. Use the fiber reactive dye first, before using the all-purpose dye, because the all-purpose dye washes out far more easily.
Better yet, dispense with the all-purpose dye altogether. You'll get much better results if you use only fiber reactive dye. Dylon Permanent dye is truly far more permanent than any sort of all purpose dye. Mixing blue Dylon Permanent dye with Dylon Permanent green will work out fine. You will also find it less trouble to dye in a single step.
While all-purpose dye bleeds forever in the laundry, unless you mail-order a special cationic dye fixative such as Retayne and apply it, fiber reactive dye such as Dylon Permanent quits bleeding as soon as you get all of the unattached extra dye washed out, and then lasts for many times more washings than all purpose dye can before fading.
For a wider range of premixed colors, mail-order a good fiber reactive dye, such as Procion MX dye, from a supplier that sells many different colors. Good sources in the US include PRO Chemical & Dye, Dharma Trading Company, and Jacquard Products. See my page of Sources for Dyeing Supplies Around the World for contact info. You can order Jacquard Products dyes through Amazon; the following page shows each of the dye colors available: Color Chart for Buying Procion MX Dyes through Amazon. If you purchase via the links on that page, your purchase will help to support this web site, at no additional cost to you.
(Please help support this web site. Thank you.)
Message: Will I have problems if I try to mix all purpose dye with fiber reactive dye to make a specific color? I was unable to find Rit Kelly Green color, so I bought some green Dylon Permanent Fabric Dye. I am going to mix it with some blue Rit dye that I already had. Will this end up working out?


It's definitely not a good idea to dye with fiber reactive dye (the Dylon® Permanent dye) and all-purpose dye (the Rit® dye) in the same step. Their requirements for attaching to the fabric are too different. The best way to combine these two dyes is to first dye with one, wash out the excess dye and chemicals, then dye with the other as an entirely different step. The Dylon Permanent dye is chemically set with a high pH, already included in the mix, whereas the Rit All Purpose dye requires high heat to set as well as it can, though it never sets very permanently. Use the fiber reactive dye first, before using the all-purpose dye, because the all-purpose dye washes out far more easily.
Better yet, dispense with the all-purpose dye altogether. You'll get much better results if you use only fiber reactive dye. Dylon Permanent dye is truly far more permanent than any sort of all purpose dye. Mixing blue Dylon Permanent dye with Dylon Permanent green will work out fine. You will also find it less trouble to dye in a single step.
While all-purpose dye bleeds forever in the laundry, unless you mail-order a special cationic dye fixative such as Retayne and apply it, fiber reactive dye such as Dylon Permanent quits bleeding as soon as you get all of the unattached extra dye washed out, and then lasts for many times more washings than all purpose dye can before fading.
For a wider range of premixed colors, mail-order a good fiber reactive dye, such as Procion MX dye, from a supplier that sells many different colors. Good sources in the US include PRO Chemical & Dye, Dharma Trading Company, and Jacquard Products. See my page of Sources for Dyeing Supplies Around the World for contact info. You can order Jacquard Products dyes through Amazon; the following page shows each of the dye colors available: Color Chart for Buying Procion MX Dyes through Amazon. If you purchase via the links on that page, your purchase will help to support this web site, at no additional cost to you.
(Please help support this web site. Thank you.)
Friday, November 09, 2007
types of dye to use for dyeing Cordura nylon motorcycle clothing
Name: Dennis
Message: Greetings. I work for a motorcycle clothing company. We make all our own textile clothing. I often get customers wanting to dye their older faded clothing. Our material is 500 weight Denier Courdra. I am not sure what type of dyes will work best. I plan to try myself by dying a hi-viz yellow jacket to Olive Drab. My jacket is old and if I mess up I am not worried. Any suggestions would be greatly appreciated. The easier the better.
Please take a look at our website. www.aerostich.com
Thank you in advance for any help you can give me to pass on the our customers.
If the Cordura nylon has not been treated with a surface finish such as Teflon, a stain-resistant finish, or any sort of water-repellent finish, then it should be easy to dye. Unfortunately, it is common for nylon to be treated with a water-repellent finish, which will repel dye, as well, preventing good dyeing. If water spilled on the fabric beads up, it will not dye well, but if it soaks in pretty quickly, chances are good that the nylon will be easy to dye. See my page "How to dye nylon or polyamide".
It's important to change only from lighter colors to darker ones, because dye is transparent and will not obscure the original color. Changing a bright yellow to olive drab should be very possible. In fact, overdyeing the yellow with black will often have this effect, since many black dyes are actually very dark navy blues. You will probably want to try a dark navy blue, I believe, in your case.
The methods used for acid dyes varies according to manufacturer's instructions, but they all involve heating the garment in a large cooking pot (which should not be used for food) with enough water that the garment can move freely when stirred. You cannot dye nylon in cold water. Heat the dyebath gradually to 205°F, just below boiling. The manufacturers of Tintex® High Temp all-purpose dye recommend the use of 1 cup (250 ml) of white vinegar per 10 liters of water when dyeing wool, silk, or nylon; do the same for any brand of all-purpose dye you use, but if you buy a better dye by mail-order, instead follow the instructions provided specifically for the line of dyes you purchase.
There are many different acid dyes.
 (See
"About Acid
Dyes".) Some are better for getting a smooth solid level color,
but are not very resistant to fading in the laundry; these are called leveling acid
dyes, or strong acid dyes. The acid dyes that are found in the mixture
of dyes in all-purpose
dyes, such as Rit® All Purpose Tint and Dye, or Tintex® Easy Fabric
dye, are this type of dye. These all-purpose dyes are convenient to
find in a local store, but they often do not perform as well as other acid dyes.
They are also rather expensive in that each box will dye only four to eight
ounces of fabric; a heavy jacket may weigh a couple of pounds and require a
number of boxes of dye. For dark colors or black, you will probably use at least
twice as much dye.
(See
"About Acid
Dyes".) Some are better for getting a smooth solid level color,
but are not very resistant to fading in the laundry; these are called leveling acid
dyes, or strong acid dyes. The acid dyes that are found in the mixture
of dyes in all-purpose
dyes, such as Rit® All Purpose Tint and Dye, or Tintex® Easy Fabric
dye, are this type of dye. These all-purpose dyes are convenient to
find in a local store, but they often do not perform as well as other acid dyes.
They are also rather expensive in that each box will dye only four to eight
ounces of fabric; a heavy jacket may weigh a couple of pounds and require a
number of boxes of dye. For dark colors or black, you will probably use at least
twice as much dye.
The very most washfast of acid dyes are the premetalized dyes found in the Lanaset series of dyes, which you will have to buy by mail-order. These are also among the more expensive dyes, costing almost as much as the little boxes of Rit dye, but their results are excellent. The very best black dye for nylon is the Lanaset Jet Black; it resists running in the laundry even when washed in hot water.

Other excellent dyes for nylon are mail-order dyes such as PRO Chemical & Dye's Washfast Acid Dyes. Not all of the dyes in this line are particularly wash-proof, but they are certainly better than the leveling acid dyes such as you find in Rit. They are fine if you wash clothing dyed with them in cool water only, after sorting by color (as well as when dry-cleaned, of course.) They are among the most economical dyes you can find anywhere for dyeing nylon. If you will be dyeing more than one item, you can buy larger jars for significant price savings. Many different colors are available in this dye line. Another line of acid dye is Jacquard Acid Dye.
(Please help support this web site. Thank you.)
Message: Greetings. I work for a motorcycle clothing company. We make all our own textile clothing. I often get customers wanting to dye their older faded clothing. Our material is 500 weight Denier Courdra. I am not sure what type of dyes will work best. I plan to try myself by dying a hi-viz yellow jacket to Olive Drab. My jacket is old and if I mess up I am not worried. Any suggestions would be greatly appreciated. The easier the better.
Please take a look at our website. www.aerostich.com
Thank you in advance for any help you can give me to pass on the our customers.
If the Cordura nylon has not been treated with a surface finish such as Teflon, a stain-resistant finish, or any sort of water-repellent finish, then it should be easy to dye. Unfortunately, it is common for nylon to be treated with a water-repellent finish, which will repel dye, as well, preventing good dyeing. If water spilled on the fabric beads up, it will not dye well, but if it soaks in pretty quickly, chances are good that the nylon will be easy to dye. See my page "How to dye nylon or polyamide".
It's important to change only from lighter colors to darker ones, because dye is transparent and will not obscure the original color. Changing a bright yellow to olive drab should be very possible. In fact, overdyeing the yellow with black will often have this effect, since many black dyes are actually very dark navy blues. You will probably want to try a dark navy blue, I believe, in your case.
The methods used for acid dyes varies according to manufacturer's instructions, but they all involve heating the garment in a large cooking pot (which should not be used for food) with enough water that the garment can move freely when stirred. You cannot dye nylon in cold water. Heat the dyebath gradually to 205°F, just below boiling. The manufacturers of Tintex® High Temp all-purpose dye recommend the use of 1 cup (250 ml) of white vinegar per 10 liters of water when dyeing wool, silk, or nylon; do the same for any brand of all-purpose dye you use, but if you buy a better dye by mail-order, instead follow the instructions provided specifically for the line of dyes you purchase.
There are many different acid dyes.

 (See
"About Acid
Dyes".) Some are better for getting a smooth solid level color,
but are not very resistant to fading in the laundry; these are called leveling acid
dyes, or strong acid dyes. The acid dyes that are found in the mixture
of dyes in all-purpose
dyes, such as Rit® All Purpose Tint and Dye, or Tintex® Easy Fabric
dye, are this type of dye. These all-purpose dyes are convenient to
find in a local store, but they often do not perform as well as other acid dyes.
They are also rather expensive in that each box will dye only four to eight
ounces of fabric; a heavy jacket may weigh a couple of pounds and require a
number of boxes of dye. For dark colors or black, you will probably use at least
twice as much dye.
(See
"About Acid
Dyes".) Some are better for getting a smooth solid level color,
but are not very resistant to fading in the laundry; these are called leveling acid
dyes, or strong acid dyes. The acid dyes that are found in the mixture
of dyes in all-purpose
dyes, such as Rit® All Purpose Tint and Dye, or Tintex® Easy Fabric
dye, are this type of dye. These all-purpose dyes are convenient to
find in a local store, but they often do not perform as well as other acid dyes.
They are also rather expensive in that each box will dye only four to eight
ounces of fabric; a heavy jacket may weigh a couple of pounds and require a
number of boxes of dye. For dark colors or black, you will probably use at least
twice as much dye.The very most washfast of acid dyes are the premetalized dyes found in the Lanaset series of dyes, which you will have to buy by mail-order. These are also among the more expensive dyes, costing almost as much as the little boxes of Rit dye, but their results are excellent. The very best black dye for nylon is the Lanaset Jet Black; it resists running in the laundry even when washed in hot water.

Other excellent dyes for nylon are mail-order dyes such as PRO Chemical & Dye's Washfast Acid Dyes. Not all of the dyes in this line are particularly wash-proof, but they are certainly better than the leveling acid dyes such as you find in Rit. They are fine if you wash clothing dyed with them in cool water only, after sorting by color (as well as when dry-cleaned, of course.) They are among the most economical dyes you can find anywhere for dyeing nylon. If you will be dyeing more than one item, you can buy larger jars for significant price savings. Many different colors are available in this dye line. Another line of acid dye is Jacquard Acid Dye.
(Please help support this web site. Thank you.)
Thursday, November 08, 2007
how can we set the dye in the bingo daubers we used to color our nylon cords for making rosaries?
Name: Seri
Message: Hello. I was making nylon rosaries for my son's class of 2nd graders. I purchased 1/8" nylon cord. I was trying to find a cheap way to dye the cord. I tried the kool-aide but was looking for something that would get the kids more involved.

I purchased bingo dabbers which worked great as far as getting the kids involved. After dabbing the rosary, we put each rosary in foil and set it in a sunny window for 4 days. (I didn't have a lot of time to test the colorfastness but was hoping this would work.) I also thought of taking the rosary in foil and warming it in the oven but then someone said they might melt. I haven't tried that yet.
I brought home the rosaries, rinsed my son's rosary and the color kept bleeding. I decided to put it in the soda ash again (room temperature) and it lost more color and was still bleeding. I did pre-soak the cords in soda ash before the kids "painted" them but there were no directions so I just used warm water for 20 minutes.
Is there something I can do make the color stay? Would the retayne stuff that you have on your web site work? I'm trying to keep this as low-cost as possible but I don't want their rosaries getting wet and coloring the kids or their clothes.
Hopefully, you have a solution. Maybe they will just have to have a solid colored rosary and do the kool-aide dying method at their own home.
Are you saying that you attempted to use soda ash to fix the pigment in bingo markers? I'm sorry, but soda ash is no use at all in
setting non-dye colors such as those found in water-soluble markers. (It is used
only in fixing a certain kind of dye, fiber reactive
dye, such as Procion MX dye; these dyes are never found in markers.)
In fact, soda ash should never be used to dye nylon, because nylon takes dye
only under acidic conditions. When using a real dye to dye nylon,
always use a little vinegar or another acid, such as citric acid, to help set
the dye. I doubt that vinegar would have helped with the bingo daubers, however,
because they contain pigment, not dye.
to fix the pigment in bingo markers? I'm sorry, but soda ash is no use at all in
setting non-dye colors such as those found in water-soluble markers. (It is used
only in fixing a certain kind of dye, fiber reactive
dye, such as Procion MX dye; these dyes are never found in markers.)
In fact, soda ash should never be used to dye nylon, because nylon takes dye
only under acidic conditions. When using a real dye to dye nylon,
always use a little vinegar or another acid, such as citric acid, to help set
the dye. I doubt that vinegar would have helped with the bingo daubers, however,
because they contain pigment, not dye.
I think that what you need to do first is wash out as much of the bingo marker ink from your nylon rosary cords as possible. You're not going to be able to use bingo markers as dye. Since they are not permanent markers, but are instead intended for use on paper, they are not suitable for any material that might ever be exposed to water. Start by soaking the cords in cool water; washing out the unwanted pigments will probably go fastest in hot water.
 I am afraid that the ink in the bingo daubers will probably not respond to Retayne as a
dye fixative. Retayne will work only on dyes that have a negative charge.
There's no reason to think that the particles of pigment used in water-soluble
marking pens will have the correct electrical charge to attract the Retayne and
be held in place by it.
I am afraid that the ink in the bingo daubers will probably not respond to Retayne as a
dye fixative. Retayne will work only on dyes that have a negative charge.
There's no reason to think that the particles of pigment used in water-soluble
marking pens will have the correct electrical charge to attract the Retayne and
be held in place by it.
A permanent marking pen, such as a Sharpie marker, should work well enough to meet your
needs. These markers are resistant to water and partially resistant to
laundering, especially if you let the cords dry for at least one month before
rinsing them or otherwise exposing them to liquid. The markers do not contain
dye and cannot be set as dye, but, unlike bingo daubers,
their pigment-containing transparent inks are relatively long-lasting. When
used to mark clothing, Sharpie pen inks last through a number of runs through
the washing machine before fading much. They are labeled as being non-toxic,
but, like all art supplies that are not made of food, should never be permitted
in children's mouths.
marker, should work well enough to meet your
needs. These markers are resistant to water and partially resistant to
laundering, especially if you let the cords dry for at least one month before
rinsing them or otherwise exposing them to liquid. The markers do not contain
dye and cannot be set as dye, but, unlike bingo daubers,
their pigment-containing transparent inks are relatively long-lasting. When
used to mark clothing, Sharpie pen inks last through a number of runs through
the washing machine before fading much. They are labeled as being non-toxic,
but, like all art supplies that are not made of food, should never be permitted
in children's mouths.
If you really want to dye the nylon cords, rather than painting them with permanent marking pens, you can do so pretty easily. Once you've gotten the water-soluble marking pen ink out of the nylon cords, you can dye them with an acid dye. The best dyes for nylon are available only by mail-order—see my FAQ page on how to dye nylon —but you will not need to use the best dyes for your project. Acid dyes are also found in food coloring, unsweetened drink
mix, and even in all-purpose
dye
Acid dyes are also found in food coloring, unsweetened drink
mix, and even in all-purpose
dye
 mixtures
such as the Rit® or Tintex® brand dyes you can find at your local
pharmacy. You cannot set them in a sunny window, but instead will need to
actually cook the cords in hot water into which you have dissolved your dyes. If
you are going to be using a cooking pot, you should use only food coloring or
Kool-aid type drink mix, because textile dyes
such as Rit dye should never be used in the pots you use to prepare food. Add
one to two tablespoons of plain distilled white vinegar for every quart of water
in your dyebath. Leave the cords in the hot water as you increase the
temperature of the water until it is very hot, 205°F, which is slightly
below boiling temperature. Continue to heat the cords in the drink mix for half
an hour, then turn off the heat and allow them to cool. Rinse well in cool water
only. (Do not ever try to dye cotton with drink mix or food coloring; these food
dyes can be used only on nylon, silk, or wool.) An advantage of using drink mix
or food coloring as dyes is that you do not need to worry if you see the
children putting the cords into their mouth later.
mixtures
such as the Rit® or Tintex® brand dyes you can find at your local
pharmacy. You cannot set them in a sunny window, but instead will need to
actually cook the cords in hot water into which you have dissolved your dyes. If
you are going to be using a cooking pot, you should use only food coloring or
Kool-aid type drink mix, because textile dyes
such as Rit dye should never be used in the pots you use to prepare food. Add
one to two tablespoons of plain distilled white vinegar for every quart of water
in your dyebath. Leave the cords in the hot water as you increase the
temperature of the water until it is very hot, 205°F, which is slightly
below boiling temperature. Continue to heat the cords in the drink mix for half
an hour, then turn off the heat and allow them to cool. Rinse well in cool water
only. (Do not ever try to dye cotton with drink mix or food coloring; these food
dyes can be used only on nylon, silk, or wool.) An advantage of using drink mix
or food coloring as dyes is that you do not need to worry if you see the
children putting the cords into their mouth later.
(Please help support this web site. Thank you.)
Message: Hello. I was making nylon rosaries for my son's class of 2nd graders. I purchased 1/8" nylon cord. I was trying to find a cheap way to dye the cord. I tried the kool-aide but was looking for something that would get the kids more involved.

I purchased bingo dabbers which worked great as far as getting the kids involved. After dabbing the rosary, we put each rosary in foil and set it in a sunny window for 4 days. (I didn't have a lot of time to test the colorfastness but was hoping this would work.) I also thought of taking the rosary in foil and warming it in the oven but then someone said they might melt. I haven't tried that yet.
I brought home the rosaries, rinsed my son's rosary and the color kept bleeding. I decided to put it in the soda ash again (room temperature) and it lost more color and was still bleeding. I did pre-soak the cords in soda ash before the kids "painted" them but there were no directions so I just used warm water for 20 minutes.
Is there something I can do make the color stay? Would the retayne stuff that you have on your web site work? I'm trying to keep this as low-cost as possible but I don't want their rosaries getting wet and coloring the kids or their clothes.
Hopefully, you have a solution. Maybe they will just have to have a solid colored rosary and do the kool-aide dying method at their own home.
Are you saying that you attempted to use soda ash
 to fix the pigment in bingo markers? I'm sorry, but soda ash is no use at all in
setting non-dye colors such as those found in water-soluble markers. (It is used
only in fixing a certain kind of dye, fiber reactive
dye, such as Procion MX dye; these dyes are never found in markers.)
In fact, soda ash should never be used to dye nylon, because nylon takes dye
only under acidic conditions. When using a real dye to dye nylon,
always use a little vinegar or another acid, such as citric acid, to help set
the dye. I doubt that vinegar would have helped with the bingo daubers, however,
because they contain pigment, not dye.
to fix the pigment in bingo markers? I'm sorry, but soda ash is no use at all in
setting non-dye colors such as those found in water-soluble markers. (It is used
only in fixing a certain kind of dye, fiber reactive
dye, such as Procion MX dye; these dyes are never found in markers.)
In fact, soda ash should never be used to dye nylon, because nylon takes dye
only under acidic conditions. When using a real dye to dye nylon,
always use a little vinegar or another acid, such as citric acid, to help set
the dye. I doubt that vinegar would have helped with the bingo daubers, however,
because they contain pigment, not dye.I think that what you need to do first is wash out as much of the bingo marker ink from your nylon rosary cords as possible. You're not going to be able to use bingo markers as dye. Since they are not permanent markers, but are instead intended for use on paper, they are not suitable for any material that might ever be exposed to water. Start by soaking the cords in cool water; washing out the unwanted pigments will probably go fastest in hot water.
 I am afraid that the ink in the bingo daubers will probably not respond to Retayne as a
dye fixative. Retayne will work only on dyes that have a negative charge.
There's no reason to think that the particles of pigment used in water-soluble
marking pens will have the correct electrical charge to attract the Retayne and
be held in place by it.
I am afraid that the ink in the bingo daubers will probably not respond to Retayne as a
dye fixative. Retayne will work only on dyes that have a negative charge.
There's no reason to think that the particles of pigment used in water-soluble
marking pens will have the correct electrical charge to attract the Retayne and
be held in place by it.A permanent marking pen, such as a Sharpie
 marker, should work well enough to meet your
needs. These markers are resistant to water and partially resistant to
laundering, especially if you let the cords dry for at least one month before
rinsing them or otherwise exposing them to liquid. The markers do not contain
dye and cannot be set as dye, but, unlike bingo daubers,
their pigment-containing transparent inks are relatively long-lasting. When
used to mark clothing, Sharpie pen inks last through a number of runs through
the washing machine before fading much. They are labeled as being non-toxic,
but, like all art supplies that are not made of food, should never be permitted
in children's mouths.
marker, should work well enough to meet your
needs. These markers are resistant to water and partially resistant to
laundering, especially if you let the cords dry for at least one month before
rinsing them or otherwise exposing them to liquid. The markers do not contain
dye and cannot be set as dye, but, unlike bingo daubers,
their pigment-containing transparent inks are relatively long-lasting. When
used to mark clothing, Sharpie pen inks last through a number of runs through
the washing machine before fading much. They are labeled as being non-toxic,
but, like all art supplies that are not made of food, should never be permitted
in children's mouths.If you really want to dye the nylon cords, rather than painting them with permanent marking pens, you can do so pretty easily. Once you've gotten the water-soluble marking pen ink out of the nylon cords, you can dye them with an acid dye. The best dyes for nylon are available only by mail-order—see my FAQ page on how to dye nylon —but you will not need to use the best dyes for your project.
 Acid dyes are also found in food coloring, unsweetened drink
mix, and even in all-purpose
dye
Acid dyes are also found in food coloring, unsweetened drink
mix, and even in all-purpose
dye
 mixtures
such as the Rit® or Tintex® brand dyes you can find at your local
pharmacy. You cannot set them in a sunny window, but instead will need to
actually cook the cords in hot water into which you have dissolved your dyes. If
you are going to be using a cooking pot, you should use only food coloring or
Kool-aid type drink mix, because textile dyes
such as Rit dye should never be used in the pots you use to prepare food. Add
one to two tablespoons of plain distilled white vinegar for every quart of water
in your dyebath. Leave the cords in the hot water as you increase the
temperature of the water until it is very hot, 205°F, which is slightly
below boiling temperature. Continue to heat the cords in the drink mix for half
an hour, then turn off the heat and allow them to cool. Rinse well in cool water
only. (Do not ever try to dye cotton with drink mix or food coloring; these food
dyes can be used only on nylon, silk, or wool.) An advantage of using drink mix
or food coloring as dyes is that you do not need to worry if you see the
children putting the cords into their mouth later.
mixtures
such as the Rit® or Tintex® brand dyes you can find at your local
pharmacy. You cannot set them in a sunny window, but instead will need to
actually cook the cords in hot water into which you have dissolved your dyes. If
you are going to be using a cooking pot, you should use only food coloring or
Kool-aid type drink mix, because textile dyes
such as Rit dye should never be used in the pots you use to prepare food. Add
one to two tablespoons of plain distilled white vinegar for every quart of water
in your dyebath. Leave the cords in the hot water as you increase the
temperature of the water until it is very hot, 205°F, which is slightly
below boiling temperature. Continue to heat the cords in the drink mix for half
an hour, then turn off the heat and allow them to cool. Rinse well in cool water
only. (Do not ever try to dye cotton with drink mix or food coloring; these food
dyes can be used only on nylon, silk, or wool.) An advantage of using drink mix
or food coloring as dyes is that you do not need to worry if you see the
children putting the cords into their mouth later. (Please help support this web site. Thank you.)
Wednesday, November 07, 2007
I used a clothes dye but I can't get the dye off my hands wondered how I would get it off help
Name: Leanne
Message: hi. I used a clothes dye but I can't get the dye off my hands wondered how I would get it off help
Message: hi. I used a clothes dye but I can't get the dye off my hands wondered how I would get it off help
This
is the topmost question on my FAQ (frequently asked
questions) page. See "I forgot to wear
gloves [or I got a hole in my glove]. How do you remove dye from hands? Should I
use bleach?" .
Don't use bleach, because it is more toxic and dangerous than the dye stains themselves. Try a pot scrubber, or just wait a day or two with normal hand-washing. A good fiber reactive dye, such as Procion MX, normally wears off within two days, because the dye does not penetrate into living skin cells, but instead stays in the surface dead cells of your skin. All-purpose dyes may take longer to wear off, because some of them probably do penetrate the skin more deeply.
Next time, wear gloves! It is foolish to use household chemicals without protection.
(Please help support this web site. Thank you.)
Don't use bleach, because it is more toxic and dangerous than the dye stains themselves. Try a pot scrubber, or just wait a day or two with normal hand-washing. A good fiber reactive dye, such as Procion MX, normally wears off within two days, because the dye does not penetrate into living skin cells, but instead stays in the surface dead cells of your skin. All-purpose dyes may take longer to wear off, because some of them probably do penetrate the skin more deeply.
Next time, wear gloves! It is foolish to use household chemicals without protection.
(Please help support this web site. Thank you.)
Tuesday, November 06, 2007
I bought some fabric dye from Dharma Trading company and I lost the directions. I want to dye a pair of jeans and I am very nervous.
Name: Lettie
Message: I bought some fabric dye from Dharma Trading company and I lost the directions. The directions that they have on their website is confusing. I want to dye a pair of jeans and I am very nervous. I need someone to help me.
Are you going to dye them a solid color, or tie-dye them?
The easiest way to dye clothing a solid color is in the washing machine. See "How can I dye clothing or fabric in the washing machine?". You will need soda ash and a large quantity of salt, as well, for that.


The jeans will lose their denim look, which is produced by white threads in one direction, woven with blue in the other direction. Overdyeing blue denim another color results in a subtle two-tone effect, since the white weft threads produce a different color, when dyed, than do the blue warp threads. If the jeans are significantly faded before dyeing, the two-tone effect may be obvious, but if they are new, the results will probably look more like a plain solid-color twill.
Dharma Trading Company sells many types of dye, suitable for different types of fiber. If you are dyeing cotton, linen, rayon, or hemp, the best choice is Procion MX dye, which they sell in many colors.
(Please help support this web site. Thank you.)
Message: I bought some fabric dye from Dharma Trading company and I lost the directions. The directions that they have on their website is confusing. I want to dye a pair of jeans and I am very nervous. I need someone to help me.
Are you going to dye them a solid color, or tie-dye them?
The easiest way to dye clothing a solid color is in the washing machine. See "How can I dye clothing or fabric in the washing machine?". You will need soda ash and a large quantity of salt, as well, for that.


The jeans will lose their denim look, which is produced by white threads in one direction, woven with blue in the other direction. Overdyeing blue denim another color results in a subtle two-tone effect, since the white weft threads produce a different color, when dyed, than do the blue warp threads. If the jeans are significantly faded before dyeing, the two-tone effect may be obvious, but if they are new, the results will probably look more like a plain solid-color twill.
Dharma Trading Company sells many types of dye, suitable for different types of fiber. If you are dyeing cotton, linen, rayon, or hemp, the best choice is Procion MX dye, which they sell in many colors.
(Please help support this web site. Thank you.)
Monday, November 05, 2007
dyeing a vintage silk kimono a fluorescent color: how does the lightfastness of these dyes compare?
Name: Helen
Message: Dear Paula,
I adore your website. I am planning on dying a vintage silk kimono a fluorescent color. Do you recommend that I use Remazol Fluorescent Yellow or Rhodamine B? The lightfastness/durability/simplicity of the dye is more important to me than the color shade.
From the Remazol data sheet [PDF] it seems that the Fluorescent Yellow has a lightfastness of 3-4. The Rhodamine has a lightfastness of 2-3 (from your lightfastness page). Can I compare these values directly even though the dyes are different?
Hi, Helen. I'm glad you're enjoying the website.
A friend sent me a sample of that Remazol fluorescent yellow, but I have not used it yet. It's a very exciting idea, because as a true fiber reactive it is far, far more washfast than an acid or basic dye, and can be used on a wider range of fibers. I am happy to see the Dystar page whose link you included, as it contains some information I had not seen before.
I don't know if the numbers for the lightfastness of different dyes are 100% comparable, since the tests were presumably done by different labs, but they are the best we have. I think that the tests used are likely to be the same for lightfastness, regardless of dye type. (This is not true for washfastness, in which acid dyes are tested under much milder conditions than fiber reactive or premetalized dyes: acid dyes are wash-tested at 105°F, Lanaset dyes at 140°F, and Remazol and other fiber reactive dyes at 205°F).
Rhodamine Red, Colour Index Acid red 52 (also known as Basic Violet 10), is noted for poor lightfastness. I am also more than a little uncomfortable about its toxicity, as it has been found to cause cancer in rats, and I have not seen any evidence demonstrating that it does not do the same in humans. Look at this Rhodamine B MSDS [PDF]: "This material is considered hazardous by the OSHA Hazard Communication Standard....Severely irritating to eyes. Irritating to skin. Toxic by inhalation. Toxic if swallowed....LABORATORY TESTS INDICATE MATERIAL MAY BE CARCINOGENIC." In contrast, Remazol dyes in general have been found to be relatively non-toxic. Remazol Luminous Yellow FL is listed as safe for use on baby clothes in Dystar's information on the Oeko-Tex Standard 100 list.
Sometimes different sources yield different lightfastness numbers for the same dye. Rhodamine
B is sold by many dye retailers; Jacquard Products has kindly made information
about their acid dyes available online. The figures they give for washfastness
and lightfastness for their 620 Hot Fuchsia are 3-4 for washfastness (in
105°F water, not hot water), and 2-3 for lightfastness, so that's
consistent with the numbers I found elsewhere at an earlier date. Pro Chemical
& Dye doesn't give numbers, but, in their printed catalog, they note for
their Washfast Acid 370, which is the same dye, that it (like their fluorescent
yellow Flavine Yellow dye) has poor lightfastness, compared to other dyes in the
Washfast Acid dye line.
Rhodamine
B is sold by many dye retailers; Jacquard Products has kindly made information
about their acid dyes available online. The figures they give for washfastness
and lightfastness for their 620 Hot Fuchsia are 3-4 for washfastness (in
105°F water, not hot water), and 2-3 for lightfastness, so that's
consistent with the numbers I found elsewhere at an earlier date. Pro Chemical
& Dye doesn't give numbers, but, in their printed catalog, they note for
their Washfast Acid 370, which is the same dye, that it (like their fluorescent
yellow Flavine Yellow dye) has poor lightfastness, compared to other dyes in the
Washfast Acid dye line.
I'm not sure how much more resistant to light fading the Remazol fluorescent may be in practical terms, but the numbers you found are hopeful. I think it would be best to treat your Remazol-fluorescent-dyed garment with care, protecting it from unnecessary light. I'd advise drying garments dyed with it indoors, rather than on a sunny clothesline, and storing them in the dark.
I like Remazol dyes very much. I have been using them on cotton, with trisodium phosphate or soda ash as fixatives. I've tested them with multi-fiber test ribbons which showed that they tend to work better on silk than on cotton under these conditions. As you probably already know, Remazol dyes can also be used on silk without soda ash or TSP, with or without acid, if you steam-set them. It seems likely to be necessary to use either steaming or the high-pH chemical in order to remove the masking group that protects Remazol dyes from reacting in the bottle. Here's a link to my page on Remazol dyes.
I would love to see the results you get in dyeing your kimono. Please consider joining the Dye Forum on my site.
(Please help support this web site. Thank you.)
Message: Dear Paula,
I adore your website. I am planning on dying a vintage silk kimono a fluorescent color. Do you recommend that I use Remazol Fluorescent Yellow or Rhodamine B? The lightfastness/durability/simplicity of the dye is more important to me than the color shade.
From the Remazol data sheet [PDF] it seems that the Fluorescent Yellow has a lightfastness of 3-4. The Rhodamine has a lightfastness of 2-3 (from your lightfastness page). Can I compare these values directly even though the dyes are different?
Hi, Helen. I'm glad you're enjoying the website.
A friend sent me a sample of that Remazol fluorescent yellow, but I have not used it yet. It's a very exciting idea, because as a true fiber reactive it is far, far more washfast than an acid or basic dye, and can be used on a wider range of fibers. I am happy to see the Dystar page whose link you included, as it contains some information I had not seen before.
I don't know if the numbers for the lightfastness of different dyes are 100% comparable, since the tests were presumably done by different labs, but they are the best we have. I think that the tests used are likely to be the same for lightfastness, regardless of dye type. (This is not true for washfastness, in which acid dyes are tested under much milder conditions than fiber reactive or premetalized dyes: acid dyes are wash-tested at 105°F, Lanaset dyes at 140°F, and Remazol and other fiber reactive dyes at 205°F).
Rhodamine Red, Colour Index Acid red 52 (also known as Basic Violet 10), is noted for poor lightfastness. I am also more than a little uncomfortable about its toxicity, as it has been found to cause cancer in rats, and I have not seen any evidence demonstrating that it does not do the same in humans. Look at this Rhodamine B MSDS [PDF]: "This material is considered hazardous by the OSHA Hazard Communication Standard....Severely irritating to eyes. Irritating to skin. Toxic by inhalation. Toxic if swallowed....LABORATORY TESTS INDICATE MATERIAL MAY BE CARCINOGENIC." In contrast, Remazol dyes in general have been found to be relatively non-toxic. Remazol Luminous Yellow FL is listed as safe for use on baby clothes in Dystar's information on the Oeko-Tex Standard 100 list.
Sometimes different sources yield different lightfastness numbers for the same dye.
 Rhodamine
B is sold by many dye retailers; Jacquard Products has kindly made information
about their acid dyes available online. The figures they give for washfastness
and lightfastness for their 620 Hot Fuchsia are 3-4 for washfastness (in
105°F water, not hot water), and 2-3 for lightfastness, so that's
consistent with the numbers I found elsewhere at an earlier date. Pro Chemical
& Dye doesn't give numbers, but, in their printed catalog, they note for
their Washfast Acid 370, which is the same dye, that it (like their fluorescent
yellow Flavine Yellow dye) has poor lightfastness, compared to other dyes in the
Washfast Acid dye line.
Rhodamine
B is sold by many dye retailers; Jacquard Products has kindly made information
about their acid dyes available online. The figures they give for washfastness
and lightfastness for their 620 Hot Fuchsia are 3-4 for washfastness (in
105°F water, not hot water), and 2-3 for lightfastness, so that's
consistent with the numbers I found elsewhere at an earlier date. Pro Chemical
& Dye doesn't give numbers, but, in their printed catalog, they note for
their Washfast Acid 370, which is the same dye, that it (like their fluorescent
yellow Flavine Yellow dye) has poor lightfastness, compared to other dyes in the
Washfast Acid dye line.I'm not sure how much more resistant to light fading the Remazol fluorescent may be in practical terms, but the numbers you found are hopeful. I think it would be best to treat your Remazol-fluorescent-dyed garment with care, protecting it from unnecessary light. I'd advise drying garments dyed with it indoors, rather than on a sunny clothesline, and storing them in the dark.
I like Remazol dyes very much. I have been using them on cotton, with trisodium phosphate or soda ash as fixatives. I've tested them with multi-fiber test ribbons which showed that they tend to work better on silk than on cotton under these conditions. As you probably already know, Remazol dyes can also be used on silk without soda ash or TSP, with or without acid, if you steam-set them. It seems likely to be necessary to use either steaming or the high-pH chemical in order to remove the masking group that protects Remazol dyes from reacting in the bottle. Here's a link to my page on Remazol dyes.
I would love to see the results you get in dyeing your kimono. Please consider joining the Dye Forum on my site.
(Please help support this web site. Thank you.)
Sunday, November 04, 2007
I have a dark purple 100% Acetate dress. Can it be dyed black? If so, how?
Name: Sharon
Message: I have a dark purple 100% Acetate dress. Can it be dyed black? If so, how? Thanks
No, this is not a good project for a dye novice. In order to dye an acetate dress, you will need to use a special kind of dye called disperse dye, the same kind of dye used for polyester. See "Dyeing Polyester with Disperse Dyes".
To dye acetate with disperse dye, you will need a very large non-aluminum cooking pot, three gallons or larger and large enough for the dress to move in freely, which you will never again use for food. This pot might cost you more than a new dress! You will need to buy disperse dye by mail order (see "Sources for Dyeing Supplies Around the World"). No other kind of dye will work; you must not use all-purpose dye, fiber reactive dye, or acid dye, as they will all wash out of acetate. Before you buy the dye, weigh the dress. You will need at least 15 grams of black disperse dye for every pound that the dress weighs. The dress will need to be heated in the dyebath, with the disperse dye and an acid such as vinegar, to the temperature of a high simmer, 205°F (96°C), and held at that temperature, stirring constantly, for half an hour to an hour.
This can work, if you are willing to devote a suitable pot to dyeing, and if your dress will survive near-boiling temperatures for an extended period of time. However, it is likely that there will be some shrinkage or damage to the acetate, which may change the fit of the dress. If the dress is marked as being dry clean only, or washable only in cool or warm water, you will not be able to dye it at all.
(Please help support this web site. Thank you.)
Message: I have a dark purple 100% Acetate dress. Can it be dyed black? If so, how? Thanks
No, this is not a good project for a dye novice. In order to dye an acetate dress, you will need to use a special kind of dye called disperse dye, the same kind of dye used for polyester. See "Dyeing Polyester with Disperse Dyes".
To dye acetate with disperse dye, you will need a very large non-aluminum cooking pot, three gallons or larger and large enough for the dress to move in freely, which you will never again use for food. This pot might cost you more than a new dress! You will need to buy disperse dye by mail order (see "Sources for Dyeing Supplies Around the World"). No other kind of dye will work; you must not use all-purpose dye, fiber reactive dye, or acid dye, as they will all wash out of acetate. Before you buy the dye, weigh the dress. You will need at least 15 grams of black disperse dye for every pound that the dress weighs. The dress will need to be heated in the dyebath, with the disperse dye and an acid such as vinegar, to the temperature of a high simmer, 205°F (96°C), and held at that temperature, stirring constantly, for half an hour to an hour.
This can work, if you are willing to devote a suitable pot to dyeing, and if your dress will survive near-boiling temperatures for an extended period of time. However, it is likely that there will be some shrinkage or damage to the acetate, which may change the fit of the dress. If the dress is marked as being dry clean only, or washable only in cool or warm water, you will not be able to dye it at all.
(Please help support this web site. Thank you.)
Saturday, November 03, 2007
lots of questions before tie-dyeing: alginate, IPA, urea chemical water, how much dye, how long to microwave
Name: Rebecca
Message: I have two basic questions - with a couple of small additions!
First, I plan to use IPA (90% from drug store) to prevent sodium alginate clumping and gelation. I will scour the t-shirts, tie when wet, immerse in the urea chemical water for about 30 minutes, then squirt the dye on. I'm assuming the IPA doesn't cause any problems down the line with the dye dissolution or reaction processes - is this true? (I'm worried that my chemical water/dye solution will not well dissolved and am trying to plan ahead.)
You must be using IPA as an abbreviation for Isopropyl Alcohol, not an abbreviation I've seen much. A small amount of alcohol will not interfere with the dye reactions at all. You don't want to try dissolving Procion MX type dye directly in undiluted alcohol, because the dye is less soluble in alcohol of any sort than in water, but that's not something you would be trying. You don't need to use more than a relatively small amount of alcohol, or vegetable oil, to suspend the alginate and remove clumps, before adding it to water to make your alginate solution.
Do not presoak your shirts in the urea chemical water! That will not work at all. In the recipes you have been studying, you soak the shirts in soda ash water! You use the urea chemical water for dissolving your dye. Instead of urea chemical water, I usually just use water with urea added (one tablespoon per cup, or 15 ml per 250 ml), or even just plain water, to dissolve the dye. The only true essentials are dye, water, soda ash, and a natural fiber to dye. Other chemicals such as urea, water softener, and alginate can be helpful, but they are not required the way that soda ash is. I recommend that you look at the entries in my hand dyeing FAQ under 'dye auxiliary chemicals'.
Alginate is completely optional. Some tie-dyers like to use it, others prefer not to. The amount used in the True Tie Dye videos is relatively small.
In order to avoid problems with dissolving dye, first add just a small amount of water (or chemical water with urea etc.) to the dye, and stir it until it forms a smooth paste. Use lukewarm water to dissolve cool water dyes such as Procion MX, as hot water may encourage the dye to hydrolyze (go bad) more quickly than you want. You may add one drop of Synthrapol or hand dishwashing detergent for particularly difficult-to-dissolve colors. The recipe for mixing alginate in alcohol, which is given on Sodium alginate, Superclear, and other dye thickeners page (http://www.pburch.net/dyeing/FAQ/alginate.shtml), calls for adding the alginate in alcohol to the chemical water. You may add the chemical water to your pasted-up dye before adding the alginate, if you prefer. Or, you can skip the alginate altogether, depending on your goals. I almost never use alginate, and prefer not to, since I like the watercolor effect. but many tie-dye artists like alginate or Superclear very much.
Second, how can I estimate how much/concentration of dye solution to prepare for tie-dyeing? I'm using the pure Procion MX-dyes recommended for beginners on your site, 100% cotton t-shirts, and following the first example in the Tie Dye 101 DVD (simple spiral). I know I need to weigh the dry shirts, but I'm not sure what concentration of dye to use to have vivid colors. (I bought a microwave at a garage sale to increase the temperature for the dye reaction since it is cold here now. I plan to heat for just 3 to 5 seconds since this is a 1200 Watt oven).
The instructions in the True Tie Dye videos work well. If you are using them, follow them as closely as you can. I myself do not use their concentrate system. Instead, for intense colors, I just mix my dye directly in water with urea (also add sodium hexametaphosphate if the water is hard) at a concentration of 2 to 4 teaspoons of dye per cup of water. Use four to eight teaspoons of black dye, because black always requires more dye.
You do not need to be precise in your dye amounts for tie-dyeing. Expect to use roughly one ounce of dye, total, per 6 shirts or per 3 yards of fabric. The only time you really need to weigh your shirts is if you are immersion dyeing and trying to get a reproducible color. Tie-dyeing is never entirely reproducible. Keep notes on how much dye you use, so you can decide afterwards whether to use more or less next time. It would make sense to make a note of the total weight of your shirts, as well, in case you are doing small shirts one time and extra-extra-large another.
If you are going to use a microwave oven, because your dyeing studio is too cold (under 70°F) or because you are in a hurry and do not want to wait overnight, you must wrap your dyed items individually in plastic. Let the dye soak into the garments before heating them, for at least fifteen minutes, or up to an hour, as otherwise you might dye only the outermost portion of each fiber in your shirts, resulting in a defect called "ring dyeing", which will result in very rapid wear and loss of color when the garments are worn. After the rest period to allow the dye to soak in, I prefer to seal the plastic bags, after first pressing out excess air. You must watch closely to stop the microwave as soon as the steam from the wet dyed garments begins to inflate the bags. You must not let the bags explode. Even more importantly, you must be sure that your garments are wet and stay wet throughout microwaving, as dry garments will catch on fire when microwaved. The plastic bag keeps the moisture in and allows you to SEE when your items have been heated enough, from the steam. However, I do not think that 3 to 5 seconds will be sufficient. Try it, then touch the outside of the plastic bag. If your dyed items in the bag are not hot, then you've accomplished nothing, and must microwave longer in order to hasten the dye reaction.
You don't have to overthink tie-dyeing. Better to start with a trial run in order to get a feel for it, using a basic recipe. The details will make more sense once you've gotten the basics down. You might want to try it without a thickener such as alginate the first time, then try it with the thickener the second time to decide which you prefer. Add Metaphos or Calgon T (sodium hexametaphosphate) to your mixtures if your water is hard; don't worry about it if your water is soft. Add urea to help keep your fabric moist while it reacts overnight, or else wrap your items in plastic: either one will retain moisture, you don't have to use both urea AND plastic bags. Don't bother using salt at all for tie-dyeing. Do not forget to use soda ash! Dissolve your dye up to one week in advance of dyeing, but don't let it contact the soda ash before you are ready to apply the dye to the fabric.
It would be good if you would join the Dye Forum to discuss your experiences with tie-dyeing. Being able to discuss your work adds a lot.
(Please help support this web site. Thank you.)
Message: I have two basic questions - with a couple of small additions!
First, I plan to use IPA (90% from drug store) to prevent sodium alginate clumping and gelation. I will scour the t-shirts, tie when wet, immerse in the urea chemical water for about 30 minutes, then squirt the dye on. I'm assuming the IPA doesn't cause any problems down the line with the dye dissolution or reaction processes - is this true? (I'm worried that my chemical water/dye solution will not well dissolved and am trying to plan ahead.)
You must be using IPA as an abbreviation for Isopropyl Alcohol, not an abbreviation I've seen much. A small amount of alcohol will not interfere with the dye reactions at all. You don't want to try dissolving Procion MX type dye directly in undiluted alcohol, because the dye is less soluble in alcohol of any sort than in water, but that's not something you would be trying. You don't need to use more than a relatively small amount of alcohol, or vegetable oil, to suspend the alginate and remove clumps, before adding it to water to make your alginate solution.
Do not presoak your shirts in the urea chemical water! That will not work at all. In the recipes you have been studying, you soak the shirts in soda ash water! You use the urea chemical water for dissolving your dye. Instead of urea chemical water, I usually just use water with urea added (one tablespoon per cup, or 15 ml per 250 ml), or even just plain water, to dissolve the dye. The only true essentials are dye, water, soda ash, and a natural fiber to dye. Other chemicals such as urea, water softener, and alginate can be helpful, but they are not required the way that soda ash is. I recommend that you look at the entries in my hand dyeing FAQ under 'dye auxiliary chemicals'.
Alginate is completely optional. Some tie-dyers like to use it, others prefer not to. The amount used in the True Tie Dye videos is relatively small.
In order to avoid problems with dissolving dye, first add just a small amount of water (or chemical water with urea etc.) to the dye, and stir it until it forms a smooth paste. Use lukewarm water to dissolve cool water dyes such as Procion MX, as hot water may encourage the dye to hydrolyze (go bad) more quickly than you want. You may add one drop of Synthrapol or hand dishwashing detergent for particularly difficult-to-dissolve colors. The recipe for mixing alginate in alcohol, which is given on Sodium alginate, Superclear, and other dye thickeners page (http://www.pburch.net/dyeing/FAQ/alginate.shtml), calls for adding the alginate in alcohol to the chemical water. You may add the chemical water to your pasted-up dye before adding the alginate, if you prefer. Or, you can skip the alginate altogether, depending on your goals. I almost never use alginate, and prefer not to, since I like the watercolor effect. but many tie-dye artists like alginate or Superclear very much.
Second, how can I estimate how much/concentration of dye solution to prepare for tie-dyeing? I'm using the pure Procion MX-dyes recommended for beginners on your site, 100% cotton t-shirts, and following the first example in the Tie Dye 101 DVD (simple spiral). I know I need to weigh the dry shirts, but I'm not sure what concentration of dye to use to have vivid colors. (I bought a microwave at a garage sale to increase the temperature for the dye reaction since it is cold here now. I plan to heat for just 3 to 5 seconds since this is a 1200 Watt oven).
The instructions in the True Tie Dye videos work well. If you are using them, follow them as closely as you can. I myself do not use their concentrate system. Instead, for intense colors, I just mix my dye directly in water with urea (also add sodium hexametaphosphate if the water is hard) at a concentration of 2 to 4 teaspoons of dye per cup of water. Use four to eight teaspoons of black dye, because black always requires more dye.
You do not need to be precise in your dye amounts for tie-dyeing. Expect to use roughly one ounce of dye, total, per 6 shirts or per 3 yards of fabric. The only time you really need to weigh your shirts is if you are immersion dyeing and trying to get a reproducible color. Tie-dyeing is never entirely reproducible. Keep notes on how much dye you use, so you can decide afterwards whether to use more or less next time. It would make sense to make a note of the total weight of your shirts, as well, in case you are doing small shirts one time and extra-extra-large another.
If you are going to use a microwave oven, because your dyeing studio is too cold (under 70°F) or because you are in a hurry and do not want to wait overnight, you must wrap your dyed items individually in plastic. Let the dye soak into the garments before heating them, for at least fifteen minutes, or up to an hour, as otherwise you might dye only the outermost portion of each fiber in your shirts, resulting in a defect called "ring dyeing", which will result in very rapid wear and loss of color when the garments are worn. After the rest period to allow the dye to soak in, I prefer to seal the plastic bags, after first pressing out excess air. You must watch closely to stop the microwave as soon as the steam from the wet dyed garments begins to inflate the bags. You must not let the bags explode. Even more importantly, you must be sure that your garments are wet and stay wet throughout microwaving, as dry garments will catch on fire when microwaved. The plastic bag keeps the moisture in and allows you to SEE when your items have been heated enough, from the steam. However, I do not think that 3 to 5 seconds will be sufficient. Try it, then touch the outside of the plastic bag. If your dyed items in the bag are not hot, then you've accomplished nothing, and must microwave longer in order to hasten the dye reaction.
You don't have to overthink tie-dyeing. Better to start with a trial run in order to get a feel for it, using a basic recipe. The details will make more sense once you've gotten the basics down. You might want to try it without a thickener such as alginate the first time, then try it with the thickener the second time to decide which you prefer. Add Metaphos or Calgon T (sodium hexametaphosphate) to your mixtures if your water is hard; don't worry about it if your water is soft. Add urea to help keep your fabric moist while it reacts overnight, or else wrap your items in plastic: either one will retain moisture, you don't have to use both urea AND plastic bags. Don't bother using salt at all for tie-dyeing. Do not forget to use soda ash! Dissolve your dye up to one week in advance of dyeing, but don't let it contact the soda ash before you are ready to apply the dye to the fabric.
It would be good if you would join the Dye Forum to discuss your experiences with tie-dyeing. Being able to discuss your work adds a lot.
(Please help support this web site. Thank you.)
Friday, November 02, 2007
boysenberry or violet MX-BR
Name: Sarah
Message: Paula.... I've been building up my stock of pure dyes, and
came across this one listed in the ProChem column: boysenberry or violet MX-BR.
I just called up there and the only thing the girl could find on her list was
MX-BRA, which they call scarlet. It is very much a red (not blueish) dye, and
not close to what I would call boysenberry or violet.
Do you have any idea which ProChem color number might be MX-BR?
THANKS! I'll also post this to the dyers' list.... cheers and
thanks
Boysenberry is listed among PRO Chemical & Dye's Procion MX dyes as "PRO MX Boysenberry 802 Violet MX-BR".
The code "Violet MX-BR" appears to be a made-up MX code, possibly from Standard Dyes which makes a habit of using incorrect MX codes (witness Standard's renaming of reactive violet 14 from violet MX-2R, which describes its color nicely, to the totally bogus violet MX-G). ProChem told me, when I asked some time ago, that they did not know what its Colour Index number was, and that they would ask their supplier, but they never got back to me. They said that it is definitely a single-hue unmixed dye, which agrees with my observations.
(Don't confuse MX codes that have different color base names! "MX-BR" is meaningless without its base name of "magenta" or "violet" or "red". For example, red MX-G has nothing in common with turquoise MX-G, except that both are dichlorotriazine (Procion MX type) dyes. See "What do the letters and numbers in the code name for a Procion MX type dye mean? " for more information on how to use these codes.)
Scarlet MX-BRA is a rather uninteresting though undeniably useful manufacturer's mix of two dyes; I believe that it is orange MX-2R combined with red MX-5B, which is a good way to mix a halo-free true red, using Procion MX type dyes. It is completely different from and unrelated to boysenberry. Boysenberry 802 is distinctly bluer than fuchsia (red MX-8B), but much redder (or pinker) than reactive violet 14 (whose proper MX code is violet MX-2R). It is a little difficult to dissolve. Surprisingly, boysenberry mixes with tangerine yellow (yellow MX-GR) to make a very good blood red.
It is my best guess that boysenberry is reactive violet 13 (magenta MX-B), but it might be reactive violet 12 (violet MX-4R) or reactive red 74 (pink MX-B), both of which are on the worldwide market for the textile industry. I don't have any samples of either for testing as a comparison.
Did you know that Aljo Manufacturing is now selling the beautiful blue-violet, blue MX-7RX (reactive blue 161), in the US? It's been unavailable here for many years, except for a brief period when it was imported by Scarlet Zebra.
(Please help support this web site. Thank you.)
Thursday, November 01, 2007
1) What are dyes? 2) What is Types of Dyes? 3) How to Prepare Dyes?
Name: Osama
Message: I have a project (an assessment at my institute) about Dyes Process.
I hope you can help me to give me some information about dyes.
Like:
1) What are dyes?
2) What are the different types of dyes?
3) How to prepare dyes?
And, give me some experiments of Dyes.(I want 10 experiments).
I hope you write me a message and send it very soon...
For your first question, you should start by looking the word up in any published dictionary or encyclopedia. If you do not have one at home, you can certainly find one in your school library, and there are also dictionaries available online. For additional information, examine my page "Fabric Paints: a different way to color fibers", to see the differences between dyes and paints.
You can learn about the different types of textile dyes by looking at my page "About Dyes".
To use dyes, look at the recipes on my website or as provided by any good dye retailer. To learn about how they are synthesized, I recommend the book "The Chemistry and Application of Dyes", edited by David R. Waring and Geoffrey Hallas; it is too expensive for a student to buy, but with luck this or a similar book might be available at a public or institutional library.
You should devise your own experiments for your assignment. You certainly should not try to get them designed for you by another person. You can find some suggestions of experiments for science fair experiments that were posted on August 29, 2007, in the Dye Forum on my website, and in the "Schoolwork" section of my Hand Dyeing Q&A blog.
(Please help support this web site. Thank you.)
Message: I have a project (an assessment at my institute) about Dyes Process.
I hope you can help me to give me some information about dyes.
Like:
1) What are dyes?
2) What are the different types of dyes?
3) How to prepare dyes?
And, give me some experiments of Dyes.(I want 10 experiments).
I hope you write me a message and send it very soon...
For your first question, you should start by looking the word up in any published dictionary or encyclopedia. If you do not have one at home, you can certainly find one in your school library, and there are also dictionaries available online. For additional information, examine my page "Fabric Paints: a different way to color fibers", to see the differences between dyes and paints.
You can learn about the different types of textile dyes by looking at my page "About Dyes".
To use dyes, look at the recipes on my website or as provided by any good dye retailer. To learn about how they are synthesized, I recommend the book "The Chemistry and Application of Dyes", edited by David R. Waring and Geoffrey Hallas; it is too expensive for a student to buy, but with luck this or a similar book might be available at a public or institutional library.
You should devise your own experiments for your assignment. You certainly should not try to get them designed for you by another person. You can find some suggestions of experiments for science fair experiments that were posted on August 29, 2007, in the Dye Forum on my website, and in the "Schoolwork" section of my Hand Dyeing Q&A blog.
(Please help support this web site. Thank you.)

