« 2011 October | Main | 2011 August »
Friday, September 30, 2011
Is Remazol Blue BB dye anionic or cationic, and what is the closest formula for this dye?
Name: Gizem
edited by David R. Waring and Geoffrey Hallas
Country or region: Turkey
Message: Hello. I'm a masters student in Ankara University/Turkey. I'm working on biological dye treatment with "Remazol Blue BB (RB 220)" dye, but couldn't find much about this dye. I want to ask, is Remazol Blue BB dye anionic or cationic, and what is the closest formula for this dye?
Remazol Blue BB appears to be widely available from dye manufacturers that are listed online, though not from smaller-scale retailers, with the generic name Colour Index reactive blue 220, and either one or the other of two CAS numbers, 128416-19-3 or 90341-71-2.
cuprate(4-), [4,5-dihydro-4-[[8-hydroxy-7-[[2-hydroxy-5-methoxy-4-[[2- (sulfooxy)ethyl]sulfonyl]phenyl]azo]-6-sulfo-2-naphthalenyl]azo]-5-oxo-1-(4-sulfophenyl)-1H- pyrazole-3-carboxylato(6-)]-, sodium. A molecular formula for this dye that was supplied on the AliBaba website by Shijiazhuang He Dye Chem is C22H15CuN4Na2O12S3, with a molecular weight of 733.10; however, even at a glance, the number of sulfur atoms indicated by the chemical name is obviously greater than are included in this formula, and the same source also indicates that the dye is insoluble in water, which seems quite unlikely, both because that would severely limit its usefulness as a textile dye, and because the sulfonyl groups mentioned in the formula are used in order to allow a dye to be soluble in water.
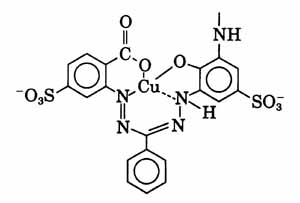
The chromophore of Remazol Blue BB includes a copper formazan complex, as listed in the book "Industrial dyes: chemistry, properties, applications", by Klaus Hunger. Other formazan dyes listed there include Colour Index reactive green 15 and Colour Index reactive blues 70, 83, 84, 104, 157, 160, 182, 202, 209, 212, 216, 218, 221, 226, 235, 260, and 263. You will probably want to search the scientific literature for all references you can find to any of these related dyes. The structure of the copper formazan choromophore should be similar to the one given by Dr. Steve Mihok, on his page "Blue Dyes", for another copper formazan dye, reactive blue 163 (CIbacron Blue F-R), though the reactive portion, which is not shown, would be quite different, since reactive blue 163 is an aminofluorotriazine dye:
Wednesday, September 28, 2011
What is the best dye to use for a polyester dress, and do you think I need to strip the colour first?
Name: Jen
Country or region: Canada
Message: I am trying to dye a dress that I had to wear at a wedding. It is 100% polyester. It is currently a peach colour. There are 2 layers of the liner underneath and the top layer is a sheer fabric. All 3 layers are polyester. What is the best dye to use and do you think I need to strip the colour first? It is relatively light. I would like to dye it as dark a purple as possible.
Is this dress washable? Don't even consider dyeing it unless you've been able to wash it without damaging it. Lined garments that are marked dry-clean-only often lose their shape altogether when washed, when the lining (whose fiber content is rarely acknowledged on the label) shrinks either more or less than the outer layers of the garment.
Assuming that you have been able to wash the dress without problems, you will need to use only a certain type of dye, called disperse dye. No other dye will work on polyester; all-purpose dye will just wash out. (See "Dyeing Polyester with Disperse Dyes".) There are several brands available. There is a low-energy type sold by Jacquard Products called iDye Poly (not to be confused with plain "iDye" without the "poly"!), which is fairly easy to acquire by mail-ordering it from a dye supplier or art supplier; in Canada, contact G&S Dye, which is located in Toronto. Additional types of disperse dye, and their auxiliary chemicals, can be ordered from PRO Chemical & Dye or from Aljo Mfg, both located in the US; Aljo, in particular, carries a wide variety of types of disperse dye, some of which are better for nylon and acetate, other truly better for polyester. iDye Poly is available in eight colors (including one violet), Prosperse Disperse Dye from ProChem in thirteen colors (including iris and lilac), and Aljo's Polyester Dyes in twenty-two different colors (including lavender, brilliant violet, and violet), plus twenty-four shades of disperse dyes optimized for acetate and nylon.
To dye polyester requires that you boil it with the disperse dye, in a very large cooking pot, large enough for the fabric to move freely, along with a horrifically smelly carrier chemical, required for full color intensity on polyester. (ProChem's new dye carrier formula might be less smelly than the one used by Jacquard Products, but I haven't tried it yet for myself; if it is as good as described, then it is very much to be preferred.) You should buy a pot that is at least five gallons in size, depending on the size of the dress. Moreover, you should not plan to reuse any dyeing pot for food, since the dyes are not safety-tested for this purpose. It's best if the dyeing pot is made of stainless steel, but an enamel canning pot is more economical, and will work fine if you are careful to always repair any chips in the enamel on the inside of the pot, using enamel paint which is not food-safe but which, fortunately, is dye-safe.
To dye a pale orange dress to a true clear purple, it will be necessary to strip the color first. If the peach color is very pale, however, and if the purple you choose is very dark and deep, then stripping the color may not be necessary. The peach underneath will give the purple a slightly browner tone, if you leave it. To try to strip the existing color, use your big dyeing pot with either Rit Color Remover, which is sodium dithionite, or Jacquard Color Remover, which is thiourea dioxide, gently cooking your dress in the very large pot of water with the color remover added, closely following the manufacturer's instructions. (See "What chemicals can be used to remove dye?".) Although Rit Color Remover can be used in the washing machine, it will often work much better when simmered in a cooking pot, and you're going to have to get the cooking pot, anyway, if you wish to dye this dress. You may need to use two or three packets of the Color Remover for the best effect, depending on the manufacturer's instructions. Never use chlorine bleach, the household bleach whose main ingredient is hypochlorite, on synthetic fibers, because it can cause permanent damage; polyester tends to acquire an ugly yellow stain, which cannot be removed. Rit Color Remover and Jacquard Color Remover are both much safer and gentler to the fiber in your dress, though the sulfur fumes they produce can be quite irritating to the lungs, especially to anyone who has asthma. Take care to have excellent ventilation, and buy a respirator that has acid gas cartridges so that you can wear it when necessary.
(Please help support this web site. Thank you.)
Monday, September 26, 2011
Is one container of black dye enough? And do I really need urea, soda ash, and synthrapol?
Name: Elizabeth
—ADVERTISEMENTS—

Country or region: U.S.
Message: Hi. I read your response on the turquoise dress and have a shirt that's similar, with 92 percent viscose and 8 percent spandex, that I also want to dye black. I was going to order the Procion MX jet black, 2/3 oz. (a) Is one container of black enough? and (b) their site says you also need the soda ash and synthropol, and that urea makes the color stronger. Do I really need these things, especially the urea? Thanks!
Procion MX fiber reactive dye is certainly an excellent choice for dyeing a viscose rayon/spandex blend.
Will you be dyeing your shirt a single solid color? If so, you should use high water ratio immersion dyeing, such as bucket dyeing or washing machine dyeing. These methods of dye application do not require urea at all.
Urea is very helpful for keeping tie-dyed or dye-painted items damp longer, because the dye-fiber reaction stops happening if the fabric dries out completely. In high water ratio immersion dyeing, or in low water immersion dyeing, there is sufficient water that you won't have a bit of trouble with drying, and so you will not need urea at all. (See my page, "What is urea for, in dyeing? Is it necessary?")
How much dye is enough? See my page, "How much Procion MX dye should I use?". What you need to do is weigh your shirt, while it's dry. The amount of fabric you have to dye, combined with the darkness of the color you want, will determine how much dye you should use. Your bathroom scale will not be able to manage something as small and light as a shirt, but your kitchen scale should do very nicely. If you don't have a kitchen scale, take your shirt to the produce department of a grocery store, or to the self-service part of a post office, and use their scale. It's very important to have an approximate idea of how much your fabric weighs.
Black is the darkest of all colors, and so you must always use much more dye powder to dye anything black than you would use for any other color. Suppose that your shirt weighs six ounces. For black, you may need as much as 10% of this dry weight, in dye powder! (You'd need far less dye for a paler color.) Since, as shown in the second table on the "How much Procion MX dye should I use?" page, you would need up to 30 grams of dye powder to dye one pound of fabric, for a six-ounce shirt, you would need about 11 grams of black dye powder.
The tiny jars sold by Jacquard Products are an excellent Procion MX fiber reactive dye, but they are very small jars. Each jar contains only 2/3 ounce of dry dye powder, which is about 18 grams. When dyeing black, one of these little jars will be sufficient for a shirt that weighs up to ten ounces.
In addition to dye, for bucket-dyeing a shirt to a single solid color, you will need soda ash or washing soda, and a large quantity of ordinary non-iodized salt, which you can buy at the grocery store. You absolutely must have either soda ash or washing soda to set the dye, and you must have the salt if you are using a large amount of water, as otherwise too much of the dye will be wasted by staying in the water and not even reaching the fiber you're dyeing. Synthrapol is an excellent detergent for pre-washing before dyeing, to remove some of the invisible stains and finishes that can interfere with dyeing, but other detergents can work, too, as long as they're not the new type that is supposed to confer stain-resistant properties.
Before you order your materials, find a good recipe for the method you're going to use, and read through the instructions to see what you will need. For solid-color dyeing, including in a bucket or in a washing machine, see my page, "How can I dye clothing or fabric in the washing machine?", which includes links to several different recipes. For mottled or multi-colored dyeing, see "How to Do Low Water Immersion Dyeing". For tie-dyeing or dye painting, see "How to Dye with Fiber Reactive Dyes".
Note that there may be a few problems in overdyeing a commercial garment. The stitching is almost certainly made of polyester, which will not take the dye, and therefore will remain the original color. Will your shirt look good black if it still has seams sewn in the original color? The answer depends very much on its style. Some garments have been pre-treated with a stain-resistant or wrinkle-resistant finish, which will limit how much color the fibers can absorb. Occasionally, a garment will be made of pieces of fabric from two different bolts, which take any added dye in different amounts, meaning that one panel may come out darker or lighter than the others. And, of course, if there is any invisible stain, whether one you made without realizing it, or one left by errors in the manufacturing process, it is possible to get a lighter region wherever the stain was originally, even if you could not see it at all before dyeing. You will be successful in redyeing a commercial garment probably nine times out of ten, if you do everything correctly, but, every now and then, something can go wrong.
(Please help support this web site. Thank you.)
Name: Ron
Country or region: USA
Message: I am looking for a white dye in a powder or dry form. I see it in liquid form but that does not help me. It has to go onto polyester using heat. I will buy a lot of this from you if you can help me. I have tried titanium dioxide but it does not stick. Thank you for your help.
Sorry, but there is no such thing. Since dye is transparent, a white dye would be colorless, just like water, and would have as little effect. A dye cannot ever cover up any darker color that is behind it. That's why you can't find a true white dye. Heat-transfer dyes for polyester are called disperse dye, but there is no such thing as white disperse dye. (See "Dyeing Polyester with Disperse Dyes". )
To cover some of the color already on a surface with white, then, you can't use a dye, but you can use an opaque pigment. However, unlike dyes, pigments do not have any ability to stick to the surface. In order to make a pigment stick, you have to mix it with something that will act as a glue. Titanium dioxide is a good white pigment, but, like any pigment, without a binder to glue it on to the fabric, it will just fall off. If you mix it with an acrylic fabric medium, then it can stick; dry heat treatment may be necessary to make the effects of the acrylic binder permanent. (Mysteriously, a web search reveals a couple of Chinese companies listing titanium dioxide as though it were a disperse dye; this must be due to a translation problem, since, as you found out the hard way, titanium dioxide simply is not a disperse dye, and cannot stick to the fiber without some sort of glue.)
The mixture of a pigment with a binder is called paint. You shouldn't use just any paint on fabric, because many of them dry to be stiff, hard, and scratchy. For painting fabric, you need to buy special fabric paint. Many fabric paints are just as transparent as dyes, so you have to look specifically for an opaque fabric paint. Some fabric paints will bind to natural fibers, but not to synthetic fibers such as polyester, so be careful to choose an opaque fabric paint whose manufacturer specifies that it will work on synthetic fibers. Jacquard Products makes a line of opaque fabric paints called Neopaque that is supposed to work on both natural and synthetic fibers. You can buy it in small jars, or special order it in quart- or gallon-size bottles, from a supplier of fabric dyes and paints. You mentioned, however, that you don't want your white "dye" to be in liquid form, which takes away the option of any sort of fabric paint. Is there any sort of heat-set glue powder that can be mixed with a pigment and then melted on to the fabric? Or are you simply trying to avoid full-fabric immersion, since you do not want a solid color? There is more than one way to solve that particular issue.
Instead of dye or paint, a third alternative is dye discharge. Many (though not all) dyes can be removed by chemically damaging them, either by oxidation or by reduction. (See "What chemicals can be used to remove dye?".) This is true of both cotton dyes and polyester dyes, but it's impossible to predict whether the dye in any particular commercially dyed garment is dischargeable, and suppliers of discharge printing inks generally say that their products are intended only for natural fibers. Chlorine bleach will discharge many dyes, but it can be damaging to polyester, forming ugly yellow stains that cannot be removed. Reductive discharge chemicals are a better choice for polyester. You apply them either by heating the polyester in water with the discharge agent, or by painting or stamping or printing them on in liquid form and using heat to activate the discharge; the specific method varies according to the chemical you choose. It cannot be applied by dry transfer methods, as disperse dyes are.
A technique you may want to look into is discharge screen printing, but the discharge agent must be one that is compatible with polyester; most screen printing discharge inks are intended for use on cotton. In theory, a discharge ink based on zinc formaldehyde sulfoxylate should work on most polyester dyes, but in practice the ones I see for sale are not recommended by their manufacturers for use on polyester. Experimentation might show that it works for some polyester dyes, but not for others, just as it works for some cotton dyes, but not others. For more specifically appropriate products, try a web search using the terms "discharge disperse dye polyester" (without the quotes).
(Please help support this web site. Thank you.)
Friday, September 23, 2011
Correcting problems with black acid dye
Name: Frank C
An ideal introduction to how to use synthetic dyes.
Country or region: USA
Message: Hiya,
I've actually come across your site a few times and I haven't really came across this issue from the topics you've discussed so I figured I'll try to bring it up to you directly and ask your opinion or experience on this:
Friends of mine and I are trying to dye a bunch of nylon with acid dye using a stovetop method. All our acid dyes so far have been Dharma's brand, and using synthrapol to wash before dyeing and after to rinse out the excess dye. Caribbean blue came out awesome, but we tried the True Black recently and it came out greyish-purple after drying. I wasn't sure if it was the dye, the nylon, or if getting a true black requires more than one dyeing round. One friend said she spoke to other dyers and they suggested dyeing a dark base color first like purple or brown and then black over it to get a true black, but that to me seems a bit odd, and in which case I don't see why we couldn't just dye using the black dye first, and then black dye again over it. Anyway, just wanted to ask if you've ever run into this issue and if you have had any luck getting an actual black dye. I'm going to try getting Jacquard's Jet Black acid dye next order to see if it turns out better, but meanwhile, I wanted to seek your advice on the issue. One of the friends also thought that pre-washing in synthrapol (since it contains alcohol) could have affected the effectiveness of the black dye if there were remnants in the fabric when it was put in the dye bath, but the Dharma rep I emailed didn't think it would affect anything. Anyway, thanks in advance if you happen to have any advice for this.
The most likely cause of your black problem is simply not using enough dye. It's very common, no matter what you are dyeing or what type of dye you are using, to get an off color, purplish or greenish or bluish or brownish, when what you want is a true black. This may be caused by using the wrong dye application recipe, or making some mistake, or to something wrong with the fiber you were dyeing, but usually it is caused by simply failing to use enough dye. Black is so much darker than any other color that you have to use a lot more dye!
You can try to correct an off-black by overdyeing with a opposite color from the opposite side of the color wheel, such as a rusty brown to correct a dark blue, but it would probably be simpler to just do another round of black, making sure to use the correct dye concentration.
How much dye powder did you use, compared to the weight of your nylon before you got it wet? Although all other colors require far less dye, Dharma says that their #413 True Black acid dye requires a huge 4% of the weight of goods you are dyeing! This is generally true for all black acid dyes, of any brand. For most acid dye colors, 1% of the weight of your fiber will be sufficient. For example, if you have a piece of nylon fabric that weighs 100 grams (a little less than a quarter of a pound), you would normally need only one or possibly two grams of dye powder to color it, or much less for a pale color, but you will need four grams of the black dye powder for that much nylon.
Quantity of dye is not the only possible issue. Different fibers will produce different colors in a given dye. A dye that makes a perfect black on wool might be slightly off-color on nylon, for example. Different acid dyes have very different properties from one another. (How many times can I use the word "different" in one paragraph?)
I don't think that traces of Synthrapol are at all likely to cause this sort of problem in acid dyeing. Many acid dyeing recipes call for Synthrapol or a similar product to be added to the dyebath itself, not just for prewashing, in order to aid in wetting the fiber, so that the dye can penetrate well. The tiny amount of alcohol in Synthrapol shouldn't do anything significant.
I really like the way Dharma lists information about each of their acid dyes. Caribbean Blue is a leveling acid dye, with the generic name of Colour Index acid blue 7, which means that it produces a very even, non-splotchy color, but it has relatively poor wetfastness, so you must take care in the laundry ever afterwards. (See my page about Leveling Acid Dyes.) This particular acid dye also has poor lightfastness, so do not line-dry it in the sunshine; always dry it indoors, if you wash it yourself, or use dry-cleaning. (See my page, "Lightfastness of Different Types of Dyes".)
Dharma's True Black acid dye, in contrast, is a premetalized dye, generic name Colour Index acid black 194, from a group of dyes that is on the opposite extreme of Leveling Acid dyes. Premetalized dyes are less good at leveling, so to get a perfectly smooth color you often add a leveling agent as called for by the recipe, but they have dramatically superior washfastness. While a leveling acid dye is likely to bleed out some even in cool water, and possibly wash out altogether in hot water, a premetalized acid dye is highly resistant to washing out, and may be able to survive being washed with even hot water, depending on the specific dye and how well it was applied. It's really a superior type of dye. Premetalized acid dyes require a higher optimal pH than the leveling acid dyes (which are also known as "strong acid dyes"), closer to a neutral pH, which means they need less acid; they are also a little more difficult to apply correctly. You may get better results by using ammonium sulfate instead of the vinegar or citric acid you might use with a leveling acid dye. It really doesn't make sense to follow the exact same recipe for dyeing with a premetalized acid dye as you would use for a leveling acid dye, given that they have such different requirements for pH. (See the chart on my page, "What is the effect of pH in dyeing? What is the optimal pH?".)
It won't hurt for you to try a different dye, though I suspect that Dharma's True Black will work perfectly well for you with some adjustment of quantities and, if necessary, tweaking of the recipe. Jacquard's Acid Black is not likely to be superior to Dharma's True Black. Unlike Dharma's True Black, Jacquard's Acid Black is a mixture of more than one color of dye, which means that it is more likely to separate out into different colors on the fiber itself, if you allow that to happen. As with Dharma's black acid dye, large quantities are essential. Jacquard Products recommends that you use three whole ounces (that's 85 grams) of their 639 Black acid dye to color just one pound of fabric or yarn! That would be six of the smallest (15 gram) jars of Jacquard Acid Dye, if you're buying them in that form, which would be an expensive way to do it, though the larger jars provide a much better deal. Be sure that you weigh whatever you are dyeing, while it is dry, and make certain that you are ordering a large enough quantity of dye!
A couple of other very popular black acid dyes are the Lanaset Jet Black and the Washfast Acid Jet Black. The latter contains Colour Index acid black 172, while the former contains a mixture of Colour Index acid black 172 with another black acid dye that does not have a Colour Index generic name. (See my Dye Forum post, "Lanaset Jet Black contains Washfast Acid Jet Black WF672 plus another black dye".) In the US, both classes of dye are sold by PRO Chemical & Dye and by Paradise Fibers; Lanaset dyes are also sold by Earth Guild. These two dyes, the Lanaset Jet Black and the Washfast Acid Jet Black, are the most-frequently recommended of the black acid dyes, in my experience. Since they are premetalized acid dyes, I would expect them to perform similarly to Dharma's, with some differences since the dye molecules are not the same. However, the special additive sold for use with the Lanaset dyes, Albegal SET, will improve leveling. You will probably get your very best black by using the Lanaset black and following PRO Chemical & Dye's instructions for "Immersion Dyeing using Lanaset/Sabraset Dyes". [PDF]
(Please help support this web site. Thank you.)
Tuesday, September 20, 2011
Could you tell me how much bleach to use and how long I should leave it?
Name: Heather
Country or region: Tennessee (U.S.)
Message: Hello,
I looked through your frequently asked questions but I couldn't find the exact answer I was looking for. I had a light pink button-up blouse that I absolutely loved, but one day I brushed up against a towel that my mother had dripped a cleaning solution on. It left two small bleach spots on the front, one the size of a dime and the other about the size of a nickel. The shirt is 100% cotton and only cost me around $15, but I really love the fit and it has a lot of sentimental value. I looked on your website and found a dyer who was willing to strip the color from the shirt and redye it back to light pink. Unfortunately, after I paid for it, the dyer returned the shirt to me in a horrible shade of hot pink (which does not look good at all with the light pink trim and buttons). To make matters worse, she obviously did not strip the color like she claimed she would because you can still see the faint spots. I am now trying to fix the shirt myself. I tried using Rit color remover on another shirt in the past with no success, so I'm hestitant to purchase more for this . I also tried linen bleach, thinking it would be a little gentler than regular bleach, but I'm not really sure I knew what I was doing. I used about two cups in a sink full of water and it didn't even turn the water pink. However, I didn't leave the shirt in the water very long so maybe that was the problem. Could you tell me how much bleach to use and how long I should leave it? Also, is linen bleach the same as regular bleach, like Clorox? Please help me! I know it sounds silly, but I'm just heartbroken over this shirt and I get teary-eyed every time I look at it or even think about never being able to wear it again. I really have my heart set on it being light pink and I've been trying to figure out how to do that since July. Any information you could give me would be appreciated. I'm desperate! Thank you for your time.
This is a sad situation. Bleach spots are very difficult to cover by dyeing, since dye is transparent and cannot completely cover a light spot in the same color as its darker surroundings. My usual suggestion is to find a close color match among the many different fabric markers sold at a good crafts or hobby store, and use it to color in the spot. See my page, "How can I fix the bleach spots on my favorite clothing?".
To remove dye, there are several available chemicals. See my page, "What chemicals can be used to remove dye?".
Sulfur-based Color Remover chemicals, such as Rit Color Remover (which is sodium dithionite) or Jacquard Color Remover (which is thiourea dioxide), work best at very high temperatures. You can try using either one in a cooking pot on your stovetop. This will work much better than using the color remover in a washing machine, since the water temperature never gets very hot in an American-made washing machine. (I always advise trying the washing machine method first anyway, since it's so much less trouble, but it sounds like you've gone beyond that with this shirt.) Rit Color Remover works extremely well on some dyes, and not nearly as well on others; it is impossible to know which dye you have until you try it. It's worth trying again with added heat, if you have not done so already, since it is much more effective that way. You should be able to find Rit Color Remover in your local fabric store, and very possibly also in a pharmacy or even at a big store like Walmart. Follow the instructions inside the box carefully!
Since bleach was able to make the spot to begin with, you might, very carefully, try using bleach to lighten the color of the blouse. While household chlorine bleach, which is based on the chemical sodium hypochlorite, can be damaging to fibers, especially synthetic fibers, it can be used on cotton if you are careful not to damage the fabric. Do not use bleach on the stovetop, since it becomes much more damaging when heated. Dilute the bleach with water in a bucket or washing machine, add the blouse, and stir it continuously (while wearing safety glasses, rubber gloves, and an apron, in case of splashes), until as much dye has been removed as it going to come out.
How much should you dilute the bleach? That depends entirely on the dye, so it's unpredictable. First try one cup of regular bleach per gallon of warm water, as recommended by The Clorox company's "Dr. Laundry" under "Lightening Blue Jeans". That is a 1:16 dilution. They say to leave it for 5 to 7 minutes, but you can allow up to 30 minutes, while watching closely for any color change, and stirring to prevent uneven bleaching. If that does not work, then you can try a stronger concentration, two cups or even four cups, but be careful to be ready to neutralize any remaining chlorine bleach immediately after washing out the blouse. Also note that the fumes from chlorine bleach are not at all healthy for your lungs, so it is best to do this out-of-doors. Rinse the bleach out thoroughly. To neutralize the bleach, use hydrogen peroxide or a thorough washing with a color-safe oxygen "bleach" such as OxyBoost; the peroxide produced by this sort of product will help to neutralize the continuing damage that can otherwise be done by the hypochlorite bleach. See my page, "How can I neutralize the damaging effects of chlorine bleach?".
It turns out that there is nothing special about Clorox Bleach Clean Linen, aside from (according to the MSDS) being a little more diluted with water, like other scented Clorox products. It is not specially effective for cleaning linen (which is a cellulose fiber, like cotton). What earns it the name of "clean linen" is merely the added fragrance, typically a cheap synthetic musk, which, to many people, after years of experiencing laundry detergents scented with cheap synthetic musk perfumes, has become synonymous with the scent of clean laundry. Personally, I prefer to get the plainest, least expensive hypochlorite-containing bleach, containing the fewest different ingredients, since I don't care for cheap perfumes. Unscented bleach has the added convenience of being usable for disinfecting water at home, in the event of a local natural disaster that contaminates the water supply, something that happens whenever water treatment plants lost power; scented bleach is not recommended for this purpose, but it's not as though you can just run out to the store and buy a new bottle of bleach under these circumstances. It's handier to make sure that you have some on hand among your laundry supplies.
If nothing else works for correcting the spot on your blouse, you will need to come to terms with owning it in a new color, whether the brighter pink or something else altogether. If you use the very easy-to-do technique of low water immersion dyeing (LWI), the resulting mottled pattern will work well for covering up the spot. So will tie-dyeing, for a different look. It won't be the same, but it might be better.
(Please help support this web site. Thank you.)
Friday, September 16, 2011
How can I overcome problems in dyeing hemp webbing?
Name: Jimena
Country or region: Peoria, IL
Message: Hello! I'm writing to you, because after reading and reading and trying different things, I still haven't obtained the result I'm looking for. I'm trying to dye hemp webbing (for now, only into black, but I would like more colors in the future). I've talked to the manufacturer that's supplying me the material and he told me it would be almost impossible for me to get a good result, but I used to get hemp webbing dyed black from another manufacturer that had a wonderful quality product, but decided not to carry the product anymore, so I know it's possible to dye natural hemp webbing. Any suggestions?
Some hemp certainly can be dyed well, because it is a cellulose fiber. This particular manufacturer, however, could be selling webbing that will not dye well. Take a sample of just a few yards, wash it, and try dyeing some of it with a good fiber reactive dye, such as Procion MX dye, following a good recipe such as one of the ones you will find on my site. If that doesn't work very well, try cleaning the hemp more carefully before dyeing, using very hot water or even boiling the hemp webbing, along with some detergent and some extra soda ash or washing soda, for extra cleaning power. Try dyeing the more carefully cleaned hemp with the same good dye, and see what happens.
There are several possible reasons why your new source of hemp webbing may not be capable of dyeing as well as those from other sources. Is it truly 100% hemp, and not part or wholly synthetic (in spite of the fiber claim made by the manufacturer)? Has it been chemically altered in a way that prevents it from reacting with dyes (as bamboo rayon and soy silk sometimes are)? Does it still contain too much unprocessed plant material, such as natural waxes? Has it had a surface finish applied that will prevent the dye from fully accessing the fiber? Problems could come from something that was applied in manufacture (e.g. spinning oils), or a resin to prevent pilling, or a stain-resistent or wrinkle-resistant treatment (though it's hard to imagine wrinkle-resistance as being big for hemp). It might be a slightly lubricating finish, to make webbing wind on and off of rolls better. Starch and other sizings can interfere badly with dyeing, in different ways. Any of these factors can make a big difference in whether a product is suitable for dyeing. Some can be removed by careful washing or boiling, but that won't work for others. Some finishes can be removed only with muriatic acid, a process that I do not want to recommend.
What kind of dye are you using now? Depending on what you are using, a change in dye might bring big improvements. Some dyes are much more effective and higher in quality than others. Do not bother to dye cellulose fibers, such as cotton or hemp, with all-purpose dyes, such as Rit dye. All-purpose dye is overpriced and diluted with large amounts of salt and detergent, and its requirement for simmering water makes it less convenient to apply, but, more importantly, it never really bonds well to the fiber, so it washes out quickly and tends to bleed color when wet. There are commercial dye fixatives that improve wetfastness, but they tend to accelerate the fading caused by sunlight.
The best choice for hemp, in most cases, is a fiber reactive dye, such as Procion MX dye. In some unusual circumstances, the use of some of the more light-resistant vat dyes may be preferable, but for most purposes, fiber reactive dyes are the best.
There are several steps to take to get the most intense color possible on hemp. Is it very tightly woven? If so, an overnight presoak in water with just a drop of liquid detergent may help the dye to penetrate. This step is often helpful when dyeing any tightly woven canvas, but is unnecessary for looser weaves that are easily penetrated by dye. For the deepest colors, whichever dye and technique you choose, you will need to be sure to follow a good recipe correctly. Dyeing with Procion MX dye should take place at a minimum of 70°F; warmer temperatures are even better. Hard water needs to be corrected by adding a specific water softening chemical, sodium hexametaphosphate, which is carried by most dye suppliers.
For a very deep, dark black, you will need to use a lot of dye powder. To determine how much dye to use, take your length of hemp webbing and weigh it, while it is dry. Your small test piece might weigh only 20 grams, while your entire spool of webbing, which you will need to unwind and tie up in loose bundles, might weigh a pound or more. However much you are dyeing at a time, you will want to use up to 10% of the dry weight of the hemp in dye powder. Much less dye is required for lighter shades, but black requires a lot of dye. If the hemp you are dyeing weighs 20 grams, you will need to use 2 grams of Procion MX dye powder; if it weighs 454 grams (that's a pound), you'll need to use 45 grams of dye powder, for the deepest darkest result. For a color that is paler than black, you might need no more than a tenth as much as that.
Will you always be wanting to dye your webbing in plain solid colors, or will you sometimes want mottled or tie-dyed combinations? There are many different ways to apply the dye, for different effects. Rainbow dyeing works great with the same recipes used for tie-dyeing. For a perfect solid color, you'll want to use a larger amount of water so that the webbing can move freely as you stir it in the dye, in which case the recipe will also call for a large quantity of salt. It's important to find a good dye recipe for the technique you're interested in, so you can avoid the mistakes that result in reduced color intensity.
(Please help support this web site. Thank you.)
Thursday, September 15, 2011
How do I dye my dark blue cotton sofas a lighter shade without using bleach?
Name: Becki
Country or region: UK
Message: I'd like to dye my dark blue cotton sofas a lighter shade. Perhaps light grey or blue. How do I remove the dye without using bleach? So far I've tried sodium percarbonate. Nothing happened. What do you suggest I use?
Use fabric, not chemicals, to change the color of your sofas. Use new cotton fabric, in whatever color you like. Buy a book on how to do your own reupholstering, or pay professionals to reupholster your sofas, or buy slipcovers for them.
Percarbonate and perborate are known as "color safe" bleaches, because the hydrogen peroxide that they liberate, when dissolved in water, is very unlikely to remove a significant amount of the color from fabric.
You can't use dye to lighten the color of anything, because dye is transparent, so the underlying color always shows through. Instead, to lighten the color of fabric, you must use a dye discharge chemical to damage the dye molecules so that they lose their color. The two main types of dye discharge agents are hypochlorite bleach, which you've already rejected (and which will cause serious damage unless you can completely rinse out and neutralize all of the remaining hypochlorite afterwards), and reductive discharges such as Color Remover, which require heating the dyed material in a container of water with the dye discharge agent. Obviously, this will not work at all for upholstery, unless you remove the fabric from the furniture first. (See "What chemicals can be used to remove dye?".)
It is possible to use an opaque fabric paint to cover a dark color, but the results will rarely look good, the amount of paint required to cover will feel slightly rough, and the large amount of opaque fabric paint that will be required will cost more than making a new slipcover. In addition, fabric paint coats only the surface of the fibers in the fabric, so it wears off relatively quickly, meaning that the results do not last nearly as long as the results of reupholstering. Most fabric paints are transparent, rather than opaque, so they cannot cover a darker color at all.
For more information, see:
Wednesday, September 14, 2011
How can I substitute sodium sulfate decahydrate for anhydrous sodium sulfate, for use as Glauber's Salt in dyeing fabric?
Name: Tiffany
Country or region: Canada
Message: I have recently come across an issue with sodium sulfate decahydrate. I recently purchased Glauber's salt from Dharma's. I ran out and my hubby bought more from the chem supply at the university he works at. Now I have discovered that Dharma's Glauber's salt is actually anhydrous sodium sulfate. I now have 1 KG ($84) of sodium sulfate decahydrate. Can I even use this? If so, how much would I use for one pound of fabric?
"Anhydrous" means that the form of the sodium sulfate molecule does not include any water molecules complexed with it. "Decahydrate" means that, as in washing soda, each molecule of the chemical has ten molecules of water attached within its crystalline structure.
According to Doug Wilson's Dyes and Dyeing Glossary [PDF], "In the dye industry, some use 'sodium sulfate' to refer to the anhydrous form, and 'Glauber’s salt' only to refer to the decahydrate form." That is not universally true, however, which is why Wilson said "some". The MSDS of the Glauber's salt sold by Dharma describes it as anhydrous, as does the description in the catalog. Other dye suppliers, such as PRO Chemical & Dye, also supply the anhydrous form.
When using Glauber's salt as a diluent for dye powders, whether to make them a standard strength or to make it easier to measure out suitable quantities for dyeing pale colors, it's good to avoid the extra water content of the decahydrate form of Glauber's salt, since the anhydrous form of sodium sulfate is useful for keeping other chemicals dry. Keeping dye powder absolutely dry is important for long storage. However, since you will most likely be using your Glauber's salt, of whichever form, only when dissolved in water, it will not matter at all which you use. You'll have plenty of water present anyway, at that point. The only question is how much of your sodium sulfate powder to measure out to use.
The decahydrate form of a substance is always larger and heavier than the anhydrous form, thanks to the presence of all those water molecules. The molecular weight of anhydrous sodium sulfate is 142.04 grams per mole, while the molecular weight of sodium sulfate decahydrate is 322.20 grams per mole (where "mole" is a certain specific number of molecules). This means that 142 grams of the anhydrous form contains the same number of sodium sulfate molecules as 322 grams of the decahydrate. 1000 grams of sodium sulfate decahydrate is equivalent to 441 grams of anhydrous sodium sulfate; 1000 grams of anhydrous sodium sulfate is equivalent to 2268 grams of sodium sulfate decahydrate.
It's most accurate to use weight rather than volume for measuring the amounts you use of dyes and dye auxiliaries; however, measuring by volume is often more convenient. The density of the anhydrous form is listed as 2.664 grams per milliliter, while the density of the decahydrate is listed as 1.464 grams per milliliter. This implies that 15 ml, or one tablespoon, of the decahydrate is equivalent to 8 ml of the anhydrous form, which is a little more than one and a half teaspoons.
How much should you use for one pound of fabric? That depends very much on what fiber you are dyeing, on what type of dye you are using, and on what method you are using to apply the dye. Find a reliable recipe, possibly one supplied by your dye retailer, and follow it closely, except for multiplying the amount of Glauber's salt by a factor of 1.8 when measuring by volume, or by a factor of 2.3 when measuring by weight, to correct for using the decahydrate form of sodium sulfate rather than the anhydrous form. I would not bother to use Glauber's salt at all for tie-dyeing cotton, for example. You can choose to substitute it for the salt (sodium chloride) in the high water ratio immersion dyeing of cotton with fiber reactive dye, using a five-gallon bucket or a washing machine. Glauber's salt is more important in dyeing wool. For dyeing wool with ProChem's Washfast Acid Dyes, you would use one tablespoon (which ProChem lists as weighing 15 grams) of anhydrous sodium sulfate while dyeing one pound of wool, or, for the same effect, substitute 34 grams (two tablespoons plus one scant teaspoon) of the decahydrate.
An interesting point about Glauber's salt is that, in addition to its important special properties for dyeing wool, it is also said to increase the color yield when dyeing with copper phthalocyanine fiber reactive dyes, such as Procion Turquoise MX-G. It will brighten the turquoise more than other colors of the same class of dye. As a result, if you substitute Glauber's salt for sodium chloride (ordinary table salt) when dyeing with color mixtures that include turquoise, you will obtain a different color than is predicted by the color chip on the chart for the dye color. Many of the different pre-mixed Procion dye colors include turquoise as an ingredient; Dharma even marks which of their premixed Procion colors contain it, in their catalog. If you care about producing a color very close in hue to the printed color chip from the catalog or the website, when using one of these pre-mixed colors, you should avoid substituting sodium sulfate for sodium chloride, as doing so will change the color, shifting the color to make it more blue. It's not a universal bluing effect, however; it will not make any other blue dye more blue, only those blue dyes that happen to be based upon the copper phthalocyanine molecule.
By the way, I think the purity of the sodium sulfate decahydrate from your husband's chemistry supply department may be higher than you need for dyeing. $84 is a lot for a kilogram of sodium sulfate decahydrate, given that G&S Dye, in Toronto, sells it for $2.75 per kilo, while Maiwa in Vancouver sells it for $8 per kilo (in Canadian dollars).
(Please help support this web site. Thank you.)
Monday, September 12, 2011
I've been searching for white or natural, viscose/rayon ribbon yarn in large hanks for dyeing
Name: Agnes
Country or region: US
Message: I've been searching for white or natural, viscose/rayon ribbon yarn in large hanks for dyeing. Can't find it at Dharma or anywhere else. Do you know a source? I know it exists because I see expensive small rolls or skeins of dyed ribbon yarn in yarn stores.
Thanks.
This is the only source I've seen for undyed viscose rayon ribbon yarn, but it's in the UK:
Name: Sharin
Country or region: Canada
Message: How soon can fresh dyed clothing be put in the dryer?
You should never put freshly dyed clothing into the dryer until after you have washed the unattached excess dye from the clothing. The excess dye must be washed out, or else it will rub off onto the inside of the dryer, making a difficult-to-clean-out mess that may ruin the next clothing you put into the dryer, if you don't do a good enough job of scrubbing it out.
There is no reason to put any freshly dyed clothing into the dryer until after the dye has been set and the clothing has been washed. Dry heat should not be used for setting dye, though it can be used to set fabric paint, after the paint has completely dried. The different methods used to set dye depend on what kind of dye you are using, and what fiber you are dyeing. As a general rule, dyes require the presence of moisture in order to bond to a textile fiber; as soon as the fabric dries completely, the reactions between the dye and the fiber used to make the clothing will cease to proceed.
After the dye has been set, and all of the excess unattached dye washed out, you are free to machine-dry freshly dyed clothing. There is no need to wait longer.
In contrast to dye, I like to let fabric paints, and fabric markers, dry longer on the fabric before washing. Fabric paints and fabric markers may look like dye, but the way that they work is completely different. The pigments in fabric paints and fabric markers do not themselves attach to the fiber, but must be glued on by the acrylic binder. Always follow the manufacturer's instructions for heat-setting fabric paints and markers, using, if recommended, either a hot iron, a transfer press, or a commercial clothes dryer (home clothes dryers don't usually get hot enough). Whether or not the manufacturer recommends heat-setting, it is often a good idea to postpone the first washing for at least a couple of weeks after you apply a fabric paint or use a fabric marker.
(Please help support this web site. Thank you.)
Friday, September 09, 2011
Where is the best place to buy supplies that can be shipped to Georgia that will have a lot of colors?
Name: Jamie
Country or region: United States
Message: My wife & sisters are dyeing shirts for a fund raiser for their mom who has Lou Gehrig's disease. Where is the best place to buy supplies that can be shipped to Georgia that will have a lot of colors? Thank you for replying!
The highest quality and easiest-to-use dyes for dyeing t-shirts are also among the least expensive, if you order them from a specialty dye supplier. I recommend that you order Procion MX type fiber reactive dyes from either PRO Chemical & Dye in Massachusetts, or from Dharma Trading Company in California. You can see additional dye retailers on my page, "Sources for Dyeing Supplies Around the World".
You can order individual dye colors, as well as the soda ash, plastic bottles, and any other ingredients or materials, or you can order all of your dyes and materials already combined into a tie dyeing kit. Both of these dye sellers can supply tie-dyeing kits, containing superior Procion MX dye and everything else you need, for anywhere from just a few shirts to over a hundred. Both companies also sell 100% cotton t-shirts that you can dye; Dharma also sells all manner of other dyeable clothes, pillow covers, book covers, and so forth.
If you try to buy locally, at a crafts store, instead of ordering online, you will find that the prices are higher and the color selection much smaller. A very good brand of tie-dyeing kit that you may find in your local fabric or hobby shop is made by Jacquard Products. When buying locally, you must be careful to avoid inferior tie-dyeing kits made with all-purpose dye, such as Rit dye, or with fabric paints; neither of these will produce results that are nearly as beautiful or long-lasting as Procion tie-dyes. Most crafts stores that sell t-shirts only carry 50% cotton/50% polyester shirts: don't try to dye these, because 100% cotton produces much better results.
(Please help support this web site. Thank you.)
Thursday, September 08, 2011
Name: Rena
—ADVERTISEMENTS—

Sodium Carbonate
Country or region: USA
Message: I understand from the FAQ that I would need to use more washing soda than the soda ash I would be able to get from a dye supplier. Rather than guesstimate how much more, I would think I could just test the ph of the solution & if it is in the range of 11 or so, it should be okay. Am I right? Thanks. I love your website.
You're correct. For dyeing with a fiber reactive dye, such as Procion MX dye, you need to use only enough washing soda, or soda ash, to get in the right pH range. See my page, "What is the effect of pH in dyeing? What is the optimal pH?".
However, surprisingly, using only just barely enough sodium carbonate to put plain water into the right pH range will not be sufficient. Since cotton itself acts as a mild acid, the pH of a very weak solution of sodium carbonate will go down after you add the cotton to it. The same is true of other cellulose-based fibers. This means that you should test your pH after adding about the same quantity of fabric (or yarn, or loose fiber) that you will normally be dyeing in that volume of water.
Fortunately, there is a wide range of sodium carbonate concentrations that will work to produce a suitable pH, so you will do fine if you add too much sodium carbonate. A nice thing about sodium carbonate, whether you buy it as soda ash (the anhydrous or monohydrate form) or as washing soda (the decahydrate form), is that you will get close to the correct pH for most fiber reactive dyes even if you use quite a lot more or less than a standard recipe calls for.
Sodium carbonate is known as a weak base, because not all of the chemical disassociates into separate ions when dissolved. If you use only a little sodium carbonate, most of it will disassociate into separate ions, but if you add a great deal of sodium carbonate, only some of it will disassociate. As you add more sodium carbonate, your pH does increase somewhat, but not nearly as quickly as it would if you used a fully-dissociating strong base, such as sodium hydroxide (also known as lye or caustic soda). Our usual recipes for dyeing with fiber reactive dyes include sufficient excess of sodium carbonate that we normally don't have to think about this very much, in either direction.
As you saw on my page about soda ash, washing soda is weaker than soda ash only because its crystals contain a good many water molecules, in addition to the sodium carbonate molecules. Soda ash contains little or no water molecules. This means that washing soda contains less sodium carbonate, whether you measure it by cups or by weight. Perhaps the most important implication of this is that washing soda should cost less than soda ash. If you see washing soda being sold for half the price per pound of soda ash, you may at first think that it's an incredible deal, when in fact it contains less sodium carbonate than soda ash does, per pound, and therefore should cost less.
Anyway, checking the pH you get, after adding a specific amount of washing soda to water and adding cotton to it, will be a good guide for whether you are using the right amount of washing soda. A pH of 11 will work nicely for all of the Procion MX dyes.
(Please help support this web site. Thank you.)
Tuesday, September 06, 2011
Is there a dye and method you can suggest for covering a stain on a wool coat that cannot be washed?
Name: Ann-Marie
—ADVERTISEMENTS—


Country or region: Ireland
Message: I have a coat labelled 'do not wash' which I have been having dry cleaned. I wish to dye it from cream to a darker colour such as brown or black. The fabric is 60%wool 30%polyester and 10%viscose. Is there a dye and method you can suggest which will allow me to dye my coat and not destroy it. I am having to dye it as there is a dark coloured stain which can not be removed even via dry cleaning. Any advice will be appreciated.
If the coat is unwearable, then go ahead and wash it. You have nothing to lose, if the coat cannot be worn with the stain. If the coat survives washing, then you can consider dyeing it. There's little point in even discussing what kind of dye to use until after you get to that point.
The thing is, you cannot dye anything without washing it. First, you have to wash any garment thoroughly before dyeing, in order to prevent stains and finishes from keeping the dye from attaching to the fiber. Secondly, you have to apply the dye in water, usually very hot water, a step that is very much like washing. And finally, you must always wash out the excess unattached dye after dyeing, as otherwise the color will rub off onto whatever touches the coat, whenever you wear it, ruining furniture and other clothing.
Washing is more destructive, for some delicate garments, than dry cleaning is, but it is also much more effective at cleaning (in addition to being far less toxic, and better for the environment). If you wash the coat, and if it survives without severe shrinkage or other damage, then you may find that the stain is gone.
However, if the stain persists even after washing, then dyeing will still not be a very good answer. All dye is transparent, which means that the stained area will inevitably end up a darker color than the surrounding dyed fabric. Sometimes dyeing a very dark color, such as black or dark deep navy blue, will cover up a stain, but it requires a lot of dye, and a very dark color. More commonly, the stain persists even after dyeing, though it may be less obvious.
If you do dye anything that consists of a wool and polyester blend, you will be unable to dye the polyester in the blend. Polyester can be dyed only with extended exposure to high heat, high enough to damage the wool. A wool dye will be able to color only 60% of the fiber in your coat's fiber blend, resulting in a color that is only slightly more than half strength.
Depending on the size and position of the stain, you might be able to sew on some sort of decoration that will cover the stain. Otherwise, your best solution to this problem will probably be to either replace the coat with a new one, or to look in secondhand stores to try to find a similar coat.
More posts on this topic:
(Please help support this web site. Thank you.)
Friday, September 02, 2011
Can I dye "polished" cotton with Procion dyes?
Name: Louie
Country or region: USA
Message: Can I dye "polished" cotton with Procion dyes?
It depends on what sort of polished cotton you have. If the "polish" is from a satin or sateen weave, then you can dye it easily. See "How can I dye satin or charmeuse?".
If, on the other hand the "polish" on your cotton is provided by a chemical finish of some sort, then it is likely to prevent the dye from reaching the cotton smoothly and evenly.
Chemical finishes are not indicated on the fiber content label. The only way to be sure of whether your polished cotton is dyeable is to cut off a small swatch (often a fabric store will give you a small sample; if not, you can buy a quarter-yard) and do a small test by trying to dye it. For one method of quickly testing with fiber reactive dye, see my August 08, 2010 blog entry about testing old dyes:
Cut the fabric up into swatches, perhaps three or six inches wide, whatever's small and convenient. Using zip-lock-type plastic freezer bags (not the storage bags which are too thin for use in storing frozen foods), place one piece of fabric in each bag. I like to use quart-sized bags for this (i.e., one-liter bags or smaller). Mix a small amount of each fiber reactive dye that you want to test with a small amount of water and pour it over a swatch of fabric in the bag. Also mix up some sodium carbonate, one teaspoon (5 ml) of soda ash per one cup (250 ml) of water, and pour some of this into each bag. Seal each bag, pressing out most of the air. Do you have a microwave oven? If so, you can microwave the bags, all at once, inside a dish of some sort, watching closely until the bags puff up with steam; stop the microwave before they can explode. The bags will slump down as the steam condenses again. Repeat this if you want to be sure the dye's good and hot, then allow to cool at room temperature. If you do not have a microwave oven, then fill a bucket or a sink with hot water, at least 60°C (that's 140°F), or hotter, heating the water on the stove if necessary, then place the sealed bags into the hot water and leave them for an hour or so. The extra heat from the waterbath or the microwave oven speeds up the dye reactions, to make the test more practical, so you don't have to wait until the next day to get your results. Drimarene K dyes like extra warmth more than Procion MX dyes do, but I have used this test for Procion dyes many times. After the bags have cooled, rinse the swatches out with cool water (a colander is handy to prevent small slips of fabric from going down the drain), then with the hottest water you have available, to see how much dye remains in the fabric after the hot water has removed as much as possible of the unattached, unbonded dye. You can place all of the swatches in a net lingerie bag to do this in the washing machine, or, for greatest efficiency, you can even pour boiling water over the fabric.
Though the above method was designed for testing dyes rather than fabric, you can adapt it to get a quick idea of whether a fabric appears to be dyeable, as long as you take care to wash the dye out thoroughly afterwards, using very hot water. (Don't forget the soda ash fixative when you apply the dye!) Sometimes fabric appears nicely dyed until the wash-out, and then it turns out that none of the dye is actually attached to the fiber, so it all washes out.
(Please help support this web site. Thank you.)
Thursday, September 01, 2011
Does longer cooking time in the dye bath mean better lightfast results?
Name: Lawanna
includes information on lightfastness of some dyes
Country or region: USA
Message: I have a question about lightfastness and acid dyes. I dye wool for rug hooking. Does it matter how long you "cook" the wool in the dye bath? Does longer cooking time mean better lightfast results?
Inadequately fixed dye can be less lightfast than it would be if properly fixed. However, trying to fix your dye extra-well by cooking it longer than needed will not improve lightfastness.
Different dyes have dramatically different susceptibilities to light-fading. It matters a great deal exactly which dye colors, and from which brands, you choose. Some classes of dyes tend to be more light-resistant than others, as well; for example, most vat dyes are extremely light-resistant, while all basic dyes, when used on natural fibers such as wool, show extraordinarily poor lightfastness; some of these basic dyes are including among the little jars of dye that are pre-mixed for use in silk painting. The different dyes within a single group of dyes also vary quite a bit in their lightfastness. As a rule, however, all of the fluorescent dyes that you can find from any class of dyes (these are the dyes that seem to glow when ultraviolet light from a blacklight hits them), are highly prone to light-fading. Some food coloring dyes, including some of those found in Kool-aid, tend to be relatively poor in lightfastness.
Take a look at my page, "Lightfastness of Different Types of Dyes". (It's in the Frequently Asked Questions section of my website.) Read the material at the top, then scroll down to the section that lists some specific acid dyes, looking under the column "light", which lists manufacturers' information on lightfastness. The scale runs from 1 to 8, where 1 indicates that a dye is highly vulnerable to light damage, while 8 indicates that it resists damage due to light as well as any textile dye can be expected to.You will see that the leveling acid dye Acid Blue 7, which is sold by Jacquard Products among the Jacquard Acid Dyes as 624 Turquoise, and by PRO Chemical & Dye among their WashFast Acid Dyes as 478 Turquoise, has an extremely poor lightfastness rating of either 2 or 1, depending on which manufacturers' information you consult.
Many dyes, especially among those made for wool, are proprietary mixtures whose individual dyes are a trade secret, so that you cannot look them up. I recommend that, if you want to be able to look up information about your dyes, you give a preference to those whose identities are honestly shared by their sellers, such as ProChem's WashFast Acid Dyes or Jacquard Acid Dyes. You will not be able to look up lightfastness information from other sources if you use Landscape Acid Dyes or Cushing Acid Dyes or Rit All-Purpose Dyes. In some cases, you may be able to obtain lightfastness information from your supplier, if they are exceptionally responsible, but this is unlikely for most dye mixtures.
Alternatively, you can dye small swatches of fabric or bundles of yarn and do your own testing, by cutting your test samples in half and hiding one half of each sample in a box or drawer where they are protected from light, and exposing the other pieces to bright sunlight over the course of many days. It will become obvious which dyes are especially prone to fading. Some dyes are protected if screened from the sun by ultra-violet-protective glass, but many are damaged by visible light alone. Ultraviolet-protective sprays are often completely ineffective.
Below is some information extracted from my lightfastness page for some of the Washfast Acid Dyes. Keep in mind the interpretations of the numbers given for lightfastness ratings. A figure of 7-8 is excellent; 6 indicates very good lightfastness; 4-5 is fair; 2-3 is poor; and 1 is very poor. (Here's a link to a table with a more detailed explanation of what these numbers mean.)
Table I. Lightfastness ratings for Washfast Acid Dyes.
| Acid Yellow 7 | 107A Flavine Yellow G | 1-2 |
| Acid Yellow 19 | 119 Sun Yellow | 5 |
| Acid Red 138 | 338 Magenta | 4-5 |
| Acid Red 151 | 351 Bright Red | 4 |
| Acid Red 266 | 366 Red | 6 |
| Acid Red 52 | 370 Rhodamine Red B | 2-3 |
| Acid Red 131 | 390 Polar Red | 4-5 |
| Acid Blue 113 | 413 Navy | 5 |
| Acid Blue 25 | 425c National Blue | 5-6 |
| Acid Blue 40 | 440 Bright Blue | 6 |
| Acid Blue 7 | 478 Turquoise | 2 or 1 |
| Acid Blue 90 | 490 Brilliant Blue | 1-2 |
| Acid Violet 17 | 817 Brilliant Violet | 1 |
Unfortunately, I am lacking data for the following Washfast Acid Dyes, as well as all of the pre-mixed colors:
Table II. Washfast Acid Dyes whose generic names are known, but for which I lack lightfastness numbers.
| Acid Yellow 135 | 135 Yellow | ? |
| Acid Yellow 199 | 199c Golden Yellow | ? |
| Acid Orange 33 | 233 Bright Orange | ? |
| Acid Red 249 | 349 Fuchsia | ? |
| Acid Black 172 | 672 Jet Black | ? |
| Acid Green 25 | 725 Forest Green | ? |
Jacquard Products very helpfully makes lightfastness and washfastness information available for their Jacquard Acid Dyes, except for the premixed colors, on their website (on a scale of 1-7, they say, rather than the usual scale of 1 to 8, probably because of the fact that no acid dye has a lightfastness rating of 8):
Table III. Lightfastness ratings for Jacquard Acid Dyes.
| 601 Yellow Sun | Acid Yellow 49 | 5-6 |
| 602 Bright Yellow | Acid Yellow 19 | 5 |
| 603 Golden Yellow | Acid Yellow 219 | 7 |
| 604 Burnt Orange | Acid Orange 116 | 5-6 |
| 606 Deep Orange | ? | 7 |
| 610 Burgundy | Acid Red 299 | 5-6 |
| 614 Violet | Acid Violet 43 | 5-6 |
| 617 Cherry Red | Acid Red 266 | 6 |
| 620 Hot Fuchsia | Acid Red 52 | 2-3 |
| 621 Sky Blue | Acid Blue 129 | 4-5 |
| 622 Sapphire Blue | Acid Blue 25 | 4-5 |
| 623 Brilliant Blue | Acid Blue 62 | 4 |
| 624 Turquoise | Acid Blue 7 | 1 |
| 625 Royal Blue | Acid Blue 324 | 5-6 |
| 626 Navy Blue | Acid Blue 113 | 7 |
| 631 Teal Acid | Green 25 | 6 |
Clearly, if you are concerned about lightfastness (as every dyer should consider being), you will do better to choose ProChem's Sun Yellow instead of Flavine Yellow, ProChem's Red or Polar Red or Bright Red instead of Rhodamine Red, and Bright Blue or National Blue instead of ProChem's Turquoise or Brilliant Blue. Similarly, Jacquard's Sky Blue, Sapphire Blue, or Brilliant Blue are to be preferred to their Turquoise, and their Cherry Red to their Hot Fuchsia. Jacquard's Violet is far more light-resistant than ProChem's Brilliant Violet, and Jacquard's Golden Yellow is significantly more light-resistant than any of the yellows in the Washfast Acid Dye line, but some of the other colors in the ProChem line are better than some colors in the Jacquard line.
(Please help support this web site. Thank you.)
|
|