« 2007 October | Main | 2007 August »
Friday, September 28, 2007
I am interested in tie dying some doctor white coats, but they are 80% polyester/20% cotton. Will that work?
Sunday, September 23, 2007
How do you dispose of unused quantities of Procion MX dye left over in the squirt bottles?
Saturday, September 22, 2007
How do I dye clothes to make them NOT DULL like is there a low gloss to add? I dyed them in a large load with black rit and they all got a little darker but everything looks dull or faded still.
Tuesday, September 18, 2007
what kinds of dye powder can be used for silk painting, to avoid the cost of shipping liquids?
Monday, September 17, 2007
I am trying to garment dye just a few pieces for sample purposes. Do you know of a recipe for this?
Thursday, September 13, 2007
What is the chemical structure of reactive red 3:1?
Monday, September 10, 2007
I am interested in dying some tagua nut slices to use as jewelry. I want something that is safe to wear next to the skin...nothing toxic!
Sunday, September 09, 2007
could you explain to me what "exhaust" the dye means? Am I to understand the dye that has been mixed with vinegar becomes exhausted and then has little impact on the environment when I dump it out?
Saturday, September 08, 2007
using different dirts from all over the state to color a quilt
Friday, September 07, 2007
my twin sons want to tie dye shirts at the party as a party favor
Thursday, September 06, 2007
How can I remove the bleach I used to clean mold off of an upholstered chair?
Tuesday, September 04, 2007
I recently bought a cream dress made from 100% Polyester. I wore it the once to a party but when I got home I noticed a huge stain on the back which i can't seem to wash out.
I am interested in tie dying some doctor white coats, but they are 80% polyester/20% cotton. Will that work?
Name: Bruce
Message: I am interested in tie dying some doctor white coats, but they are 80% polyester/20% cotton. Will that work? THANKS
No. You cannot use ordinary dyes on polyester, and 20% cotton is not a large enough proportion to allow the use of cotton dyes. For more information on the difficulties of dyeing polyester, see "Dyeing Polyester with Disperse Dyes".
Alternative one, my favorite, would be to buy some 100% cotton doctor's coats, or at least 80% cotton. You can even dye 50% cotton if you will be satisfied with pale pastels, but 100% cotton will produce much brighter colors. A good source for dyeable doctor's coats is Dharma Trading Company. They also sell dyeable scrubs, if you're interested.
I strongly advise you to use only fiber reactive dyes, such as Procion MX dyes. Do not use "all purpose" dyes, such as Rit® Tint and Dye or Tintex® Easy Fabric Dye, for tie-dyeing. You can buy Procion MX dyes by mail-order from the dye suppliers listed on my page of Sources for Dyeing Supplies Around the World.
Alternative two: if you already have the 80% polyester lab coats, and don't wish to buy new ones, I advise you to tie-PAINT them, instead of tie-dyeing. For the differences between fabric dyes and fabric paints, see "Fabric Paints: a different way to color fibers". Good choices for mimicking tie-dye with fabric paints would be Jacquard Products's Dye-Na-Flow and Dharma Trading Company's Dharma Pigment Dye.
A third alternative would be to use disperse dye crayons to make iron-ons to decorate your lab coats with. This can be great fun, but the results are different from tie-dyeing.
(Please help support this web site. Thank you.)
Message: I am interested in tie dying some doctor white coats, but they are 80% polyester/20% cotton. Will that work? THANKS
No. You cannot use ordinary dyes on polyester, and 20% cotton is not a large enough proportion to allow the use of cotton dyes. For more information on the difficulties of dyeing polyester, see "Dyeing Polyester with Disperse Dyes".
Alternative one, my favorite, would be to buy some 100% cotton doctor's coats, or at least 80% cotton. You can even dye 50% cotton if you will be satisfied with pale pastels, but 100% cotton will produce much brighter colors. A good source for dyeable doctor's coats is Dharma Trading Company. They also sell dyeable scrubs, if you're interested.
I strongly advise you to use only fiber reactive dyes, such as Procion MX dyes. Do not use "all purpose" dyes, such as Rit® Tint and Dye or Tintex® Easy Fabric Dye, for tie-dyeing. You can buy Procion MX dyes by mail-order from the dye suppliers listed on my page of Sources for Dyeing Supplies Around the World.
Alternative two: if you already have the 80% polyester lab coats, and don't wish to buy new ones, I advise you to tie-PAINT them, instead of tie-dyeing. For the differences between fabric dyes and fabric paints, see "Fabric Paints: a different way to color fibers". Good choices for mimicking tie-dye with fabric paints would be Jacquard Products's Dye-Na-Flow and Dharma Trading Company's Dharma Pigment Dye.
A third alternative would be to use disperse dye crayons to make iron-ons to decorate your lab coats with. This can be great fun, but the results are different from tie-dyeing.
(Please help support this web site. Thank you.)
Sunday, September 23, 2007
How do you dispose of unused quantities of Procion MX dye left over in the squirt bottles?
Name: Rebecca
Message: How do you dispose of unused quantities of Procion MX dye left over in the squirt bottles?
Dilute the dye with water as you pour it down the drain. Procion MX dyes are not considered toxic waste and will not damage your septic tank.
You can reuse the bottles for dyeing, after rinsing them and letting them dry. Procion MX dye powder is much more economical when purchased by mail-order in larger quantities.
(From the September 9, 2007 posting in this blog, links to pages that contain more information about disposing of Procion Dyes:
http://www.dharmatrading.com/info/procion_general_info.html
http://www.prochemical.com/StudioSafety.htm )
(Please help support this web site. Thank you.)
Message: How do you dispose of unused quantities of Procion MX dye left over in the squirt bottles?
Dilute the dye with water as you pour it down the drain. Procion MX dyes are not considered toxic waste and will not damage your septic tank.
You can reuse the bottles for dyeing, after rinsing them and letting them dry. Procion MX dye powder is much more economical when purchased by mail-order in larger quantities.
(From the September 9, 2007 posting in this blog, links to pages that contain more information about disposing of Procion Dyes:
http://www.dharmatrading.com/info/procion_general_info.html
http://www.prochemical.com/StudioSafety.htm )
(Please help support this web site. Thank you.)
Saturday, September 22, 2007
How do I dye clothes to make them NOT DULL like is there a low gloss to add? I dyed them in a large load with black rit and they all got a little darker but everything looks dull or faded still.
Name: Dan
Message: How do I dye clothes to make them NOT DULL like is there a low gloss to add? I dyed them in a large load with black rit and they all got a little darker but everything looks dull or faded still. In certain type of light they all look faded. Is there a clear coat to dye bath in?
You need to start over and use a much higher-quality dye. All-purpose dyes do not produce a good color intensity or brilliance, and they get more faded with every washing.
You also need to think about your fabric. You cannot dye polyester with ordinary dyes or procedures; dyeing polyester is not a job for the dye novice. For the brightest colors, you should use 100% cotton that has been mercerized, or at least some sort of 100% cotton; 100% rayon also gives good, intense colors. It is very important to avoid poly/cotton blends, as well as any clothing that has been treated to make it stain-resistant or permanent-press.
The best way to dye is to start with PFD (prepared for dyeing) clothing and use only high quality, fiber reactive dyes, such as Procion MX dye. Buy your dye by mail-order, because it is difficult to find high quality dyes locally. Look at my page listing Sources of Dyeing Supplies Around the World .
When dyeing black, you must use a larger amount of dye than other colors, as well. You should use two to four times as much black dye as you would use of another color. Black all-purpose dye will not work well even in larger concentrations, though, because it is a relatively poor quality dye.
If what you really want is a shine, rather than a deep intense dark black, you will need to use fabric paint instead of dye. You could get some nice effects by dyeing with Procion MX dye and then applying a high quality fabric paint of the same color on top. See "Fabric Paints: a different way to color fibers", for more information about the difference between paint and dye. However, fabric paint tends to wear off clothing much more quickly than dye, and is not as suitable for deep intense colors.
(Please help support this web site. Thank you.)
Message: How do I dye clothes to make them NOT DULL like is there a low gloss to add? I dyed them in a large load with black rit and they all got a little darker but everything looks dull or faded still. In certain type of light they all look faded. Is there a clear coat to dye bath in?
You need to start over and use a much higher-quality dye. All-purpose dyes do not produce a good color intensity or brilliance, and they get more faded with every washing.
You also need to think about your fabric. You cannot dye polyester with ordinary dyes or procedures; dyeing polyester is not a job for the dye novice. For the brightest colors, you should use 100% cotton that has been mercerized, or at least some sort of 100% cotton; 100% rayon also gives good, intense colors. It is very important to avoid poly/cotton blends, as well as any clothing that has been treated to make it stain-resistant or permanent-press.
The best way to dye is to start with PFD (prepared for dyeing) clothing and use only high quality, fiber reactive dyes, such as Procion MX dye. Buy your dye by mail-order, because it is difficult to find high quality dyes locally. Look at my page listing Sources of Dyeing Supplies Around the World .
When dyeing black, you must use a larger amount of dye than other colors, as well. You should use two to four times as much black dye as you would use of another color. Black all-purpose dye will not work well even in larger concentrations, though, because it is a relatively poor quality dye.
If what you really want is a shine, rather than a deep intense dark black, you will need to use fabric paint instead of dye. You could get some nice effects by dyeing with Procion MX dye and then applying a high quality fabric paint of the same color on top. See "Fabric Paints: a different way to color fibers", for more information about the difference between paint and dye. However, fabric paint tends to wear off clothing much more quickly than dye, and is not as suitable for deep intense colors.
(Please help support this web site. Thank you.)
Tuesday, September 18, 2007
what kinds of dye powder can be used for silk painting, to avoid the cost of shipping liquids?
Name: Val
Message: I'm leading humanitarian projects in Africa (teaching hand craft skills to unemployed women) Because of the shipping costs involved, I am considering Acid dyes (less weight as I am not paying to ship water :) ) for silk painting rather than the premixed type like Jacquard. Can you think of major down sides to using this type product? Thanks if you can help !
Jacquard Products sells many different types of dyes, most of them in powdered form. You must be referring to either the Green Label Silk Colors or the Red Label Silk Colors, both of which are sold in liquid form. Red Label Silk Colors are Remazol-type fiber reactive dyes, premixed with water to strengths of around 12% or less. Green Label Silk Colors are the same dyes as Red Label Silk Colors, but with some auxiliary chemicals added, and sold at half the concentration, i.e. 6% strength or less.
If you buy Remazol dyes from a different source, you can get them in powdered form, instead of liquid, and thus save on postage. There are also other good alternatives for buying powdered dye, such as acid dye. Your most economical option, in any case, would be to buy fairly large jars of dye powder, at least eight ounces of dye powder (that is, approximately one quarter of a kilogram), since dyes cost less when purchased in these quantities than when purchased in smaller jars.
There are several different types of dye that are popular for silk painting. The most popular in books about how to do silk painting appear to be the French silk dyes, a group that includes the brands Dupont, Pebeo, Ateliers Creatief Kniazeff, and Sennelier Tinfix; these are almost certainly made with both acid dyes and basic dyes. They are never sold in powdered form, only premixed with water and other chemicals, so they are not suitable for your needs. It is impossible to know which dyes are included in each color, because the makers of the French dyes are extraordinarily secretive about their products. (For an interesting story about just how secretive they are, check out the August 30, 2007 entry in my Hand Dyeing Q&A blog, "Did Sennelier Tinfix Silk Dyes cause my wife's hyperthyroidism?".) The French silk dyes require extensive steaming, as much as three hours, to set the dye permanently, and in some cases they are less light-resistant than other types of dyes.
Two different types of fiber reactive dye which are popular for silk painting are Remazol and Procion H. Procion H dyes are similar in structure to the Procion MX dyes that are popular for tie-dyeing, but, unlike Procion MX dyes, the Procion H dyes require steaming to set the dye. Both Remazol and Procion H require something like thirty minutes of steaming, instead of the three hours required by the French dyes. You can buy them in powdered form to save on shipping, and then mix up enough dye solution in water to meet your needs for a full year, instead of having to mix up fresh dye solutions every month or two as you would with Procion MX dyes, which go bad more quickly. This is particularly useful if it takes you some trial and error to get your dye paint mixtures just right. Both Procion H and Remazol dyes have an additional advantage in that they can also be used on cotton, linen, and rayon, not only on silk.
To buy powdered Procion H dyes, you can order your dyes from PRO Chemical & Dye in the US, which will ship internationally, or from Synthesia dyes in the Czech Republic, if they will do mail-order. To buy powdered Remazol dyes, order from Batik Oetoro or from KraftKolour in Australia, both of which ship internationally, or from Granat Farvekompagniet, in Denmark. There is contact information for each of these dye companies on my page of Sources for Dyeing Supplies Around the World.
Batik Oetoro also sells Drimarene K dyes, which are similar to Remazol and Procion H dyes, and they sell Drimalan F dyes, which they recommend for silk painting. They have detailed instructions for using Drimalan F dye powder to prepare silk paints. Drimalan F is used like an acid dye, although it is actually not an acid dye at all; it is a special class of fiber reactive dye used only on wool, silk, and nylon. It has unusually good washfastness, compared to acid dyes, as a result. Prices are high, but less of the dye is required to make your dye paint, only 10 to 20 grams per liter of dye paint.
You can buy basic dyes in powdered form, as well. Aljo Dyes in New York sells them as "alcohol/water dyes" for this purpose. I tend to avoid basic dyes because some of them are more hazardous than fiber reactive dyes, but these dyes are almost certainly used in some of the French Silk dyes, which may be more toxic than artists commonly believe. The colors of basic dyes tend to be very bright, but particularly susceptible to light fading.
The different colors of acid dyes are more variable in their properties than dyes within a group of fiber reactive dyes. There are many different types of acid dyes. Be sure to buy only those which are recommended for use together, as some acid dyes are incompatible with other acid dyes. Buying all of your acid dyes from one dye retailer is one way to avoid this problem.
In general, acid dyes will be less washfast, that is, less resistant to fading from washing, than fiber reactive dyes are. They do not allow the option of use on cellulose fibers such as cotton. They may require longer steaming than the steam-set fiber reactive dyes. However, none of these are very significant drawbacks against their use in silk painting, for most situations. Some people say that acid dyes will produce brighter, more intense colors than other types of dyes. They last well even after being dissolved in water.
In all cases, mixing your own silk paint from acid dyes or fiber reactive dyes is less convenient than buying dyes that are premixed with water, since you must experiment a little to find what strength of dye mixture is best for your purposes. Be sure to keep careful records of exactly how much dye you mix, and what you mix it with, for each of your colors, so that you will find it easy to reproduce your successes. Instead of using measuring spoons to portion out your dye, it will be very helpful for you to use a small scale to weigh out exactly the same amount of dye each time. Dye strength is standardized by weight, not volume, so results are not very reproducible if you do not measure by weight. Try to find a balance or scale that can easily weigh less than 20 grams. Use small pieces of waxed paper or foil to hold the dye that you are weighing out, to reduce contamination of your scale, and to keep your dyes from contaminating one another. Do not use the same scale for dyes that you use for foods.
If you buy a very bright pure yellow, a bright cyan/turquoise, and a bright magenta/fuchsia, you will be able to mix a wide range of colors. You should also buy a black dye. Buying additional unmixed single-color dyes is not a waste of money, but the printer's primary colors are the most important for you to have.
(Please help support this web site. Thank you.)
Message: I'm leading humanitarian projects in Africa (teaching hand craft skills to unemployed women) Because of the shipping costs involved, I am considering Acid dyes (less weight as I am not paying to ship water :) ) for silk painting rather than the premixed type like Jacquard. Can you think of major down sides to using this type product? Thanks if you can help !
Jacquard Products sells many different types of dyes, most of them in powdered form. You must be referring to either the Green Label Silk Colors or the Red Label Silk Colors, both of which are sold in liquid form. Red Label Silk Colors are Remazol-type fiber reactive dyes, premixed with water to strengths of around 12% or less. Green Label Silk Colors are the same dyes as Red Label Silk Colors, but with some auxiliary chemicals added, and sold at half the concentration, i.e. 6% strength or less.
If you buy Remazol dyes from a different source, you can get them in powdered form, instead of liquid, and thus save on postage. There are also other good alternatives for buying powdered dye, such as acid dye. Your most economical option, in any case, would be to buy fairly large jars of dye powder, at least eight ounces of dye powder (that is, approximately one quarter of a kilogram), since dyes cost less when purchased in these quantities than when purchased in smaller jars.
There are several different types of dye that are popular for silk painting. The most popular in books about how to do silk painting appear to be the French silk dyes, a group that includes the brands Dupont, Pebeo, Ateliers Creatief Kniazeff, and Sennelier Tinfix; these are almost certainly made with both acid dyes and basic dyes. They are never sold in powdered form, only premixed with water and other chemicals, so they are not suitable for your needs. It is impossible to know which dyes are included in each color, because the makers of the French dyes are extraordinarily secretive about their products. (For an interesting story about just how secretive they are, check out the August 30, 2007 entry in my Hand Dyeing Q&A blog, "Did Sennelier Tinfix Silk Dyes cause my wife's hyperthyroidism?".) The French silk dyes require extensive steaming, as much as three hours, to set the dye permanently, and in some cases they are less light-resistant than other types of dyes.
Two different types of fiber reactive dye which are popular for silk painting are Remazol and Procion H. Procion H dyes are similar in structure to the Procion MX dyes that are popular for tie-dyeing, but, unlike Procion MX dyes, the Procion H dyes require steaming to set the dye. Both Remazol and Procion H require something like thirty minutes of steaming, instead of the three hours required by the French dyes. You can buy them in powdered form to save on shipping, and then mix up enough dye solution in water to meet your needs for a full year, instead of having to mix up fresh dye solutions every month or two as you would with Procion MX dyes, which go bad more quickly. This is particularly useful if it takes you some trial and error to get your dye paint mixtures just right. Both Procion H and Remazol dyes have an additional advantage in that they can also be used on cotton, linen, and rayon, not only on silk.
To buy powdered Procion H dyes, you can order your dyes from PRO Chemical & Dye in the US, which will ship internationally, or from Synthesia dyes in the Czech Republic, if they will do mail-order. To buy powdered Remazol dyes, order from Batik Oetoro or from KraftKolour in Australia, both of which ship internationally, or from Granat Farvekompagniet, in Denmark. There is contact information for each of these dye companies on my page of Sources for Dyeing Supplies Around the World.
Batik Oetoro also sells Drimarene K dyes, which are similar to Remazol and Procion H dyes, and they sell Drimalan F dyes, which they recommend for silk painting. They have detailed instructions for using Drimalan F dye powder to prepare silk paints. Drimalan F is used like an acid dye, although it is actually not an acid dye at all; it is a special class of fiber reactive dye used only on wool, silk, and nylon. It has unusually good washfastness, compared to acid dyes, as a result. Prices are high, but less of the dye is required to make your dye paint, only 10 to 20 grams per liter of dye paint.
You can buy basic dyes in powdered form, as well. Aljo Dyes in New York sells them as "alcohol/water dyes" for this purpose. I tend to avoid basic dyes because some of them are more hazardous than fiber reactive dyes, but these dyes are almost certainly used in some of the French Silk dyes, which may be more toxic than artists commonly believe. The colors of basic dyes tend to be very bright, but particularly susceptible to light fading.
The different colors of acid dyes are more variable in their properties than dyes within a group of fiber reactive dyes. There are many different types of acid dyes. Be sure to buy only those which are recommended for use together, as some acid dyes are incompatible with other acid dyes. Buying all of your acid dyes from one dye retailer is one way to avoid this problem.
In general, acid dyes will be less washfast, that is, less resistant to fading from washing, than fiber reactive dyes are. They do not allow the option of use on cellulose fibers such as cotton. They may require longer steaming than the steam-set fiber reactive dyes. However, none of these are very significant drawbacks against their use in silk painting, for most situations. Some people say that acid dyes will produce brighter, more intense colors than other types of dyes. They last well even after being dissolved in water.
In all cases, mixing your own silk paint from acid dyes or fiber reactive dyes is less convenient than buying dyes that are premixed with water, since you must experiment a little to find what strength of dye mixture is best for your purposes. Be sure to keep careful records of exactly how much dye you mix, and what you mix it with, for each of your colors, so that you will find it easy to reproduce your successes. Instead of using measuring spoons to portion out your dye, it will be very helpful for you to use a small scale to weigh out exactly the same amount of dye each time. Dye strength is standardized by weight, not volume, so results are not very reproducible if you do not measure by weight. Try to find a balance or scale that can easily weigh less than 20 grams. Use small pieces of waxed paper or foil to hold the dye that you are weighing out, to reduce contamination of your scale, and to keep your dyes from contaminating one another. Do not use the same scale for dyes that you use for foods.
If you buy a very bright pure yellow, a bright cyan/turquoise, and a bright magenta/fuchsia, you will be able to mix a wide range of colors. You should also buy a black dye. Buying additional unmixed single-color dyes is not a waste of money, but the printer's primary colors are the most important for you to have.
(Please help support this web site. Thank you.)
Monday, September 17, 2007
I am trying to garment dye just a few pieces for sample purposes. Do you know of a recipe for this?
Name: david
Message: Hello,
I dont know if you check this. But here goes. I am trying to garment dye just a few pieces for sample purposes. Do you know of a recipe for this? Or is there one on this site that I couldn't find?
The easiest way to get a solid color when you dye garments is to use a top-loading washing machine; for very small pieces, totaling no more than one pound by weight, you can use a three-gallon bucket and spend most of an hour stirring your dyebath by hand. For garment dyeing cotton or rayon garments with fiber reactive dye, you will need soda ash, salt, and dye (such as Procion MX or Remazol or Cibacron F or Drimarene K). Avoid the use of all-purpose dyes, and avoid synthetic fiber blends such as polyester. This method works well for garments that are mostly cotton with just a little Lycra spandex.
For more information, see "How can I dye clothing or fabric in the washing machine?", and be sure to follow the links to study the various recipes.
(Please help support this web site. Thank you.)
Message: Hello,
I dont know if you check this. But here goes. I am trying to garment dye just a few pieces for sample purposes. Do you know of a recipe for this? Or is there one on this site that I couldn't find?
The easiest way to get a solid color when you dye garments is to use a top-loading washing machine; for very small pieces, totaling no more than one pound by weight, you can use a three-gallon bucket and spend most of an hour stirring your dyebath by hand. For garment dyeing cotton or rayon garments with fiber reactive dye, you will need soda ash, salt, and dye (such as Procion MX or Remazol or Cibacron F or Drimarene K). Avoid the use of all-purpose dyes, and avoid synthetic fiber blends such as polyester. This method works well for garments that are mostly cotton with just a little Lycra spandex.
For more information, see "How can I dye clothing or fabric in the washing machine?", and be sure to follow the links to study the various recipes.
(Please help support this web site. Thank you.)
Thursday, September 13, 2007
What is the chemical structure of reactive red 3:1?
Name: hiren
Message: i required to know what is the chemical structure of reactive red 3:1 as i am not able to get the latest color index database. so, request you to sir, please if you have than please give me.
I don't have ready access to the Colour Index, either. Have you tried a web search? It's easy to find the CAS number for this dye, which is 23211-47-4, and with that you can find a great deal of additional information. Brand names under which "reactive red 3:1" or "reactive red 3" are sold appear to include Tulactiv Red P4BN and Brilliant Red K-2BP. The molecular formula is C25H15ClN7Na3O10S3, and the full chemical name is 2,7-Naphthalenedisulfonic acid, 5-((4-chloro-6-(phenylamino) -1,3,5-triazin-2-yl)amino)-4- hydroxy-3-((2-sulfophenyl)azo)-, trisodium salt.
The chemical structure for Colour Index reactive red 3 is listed online in US patent 4016149 as follows: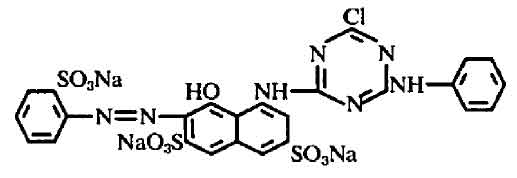
This structure is also given on page 190 of John Shore's excellent book, Cellulosics Dyeing, which is available for the very reasonable price of £9 when ordered directly from the Society of Dyers and Colourists in the UK. This dye is a 2-amino-4-chloro derivative of reactive red 1 (Procion Red MX-2B); the single chlorine makes it much less reactive, so it requires fixation temperatures of 70°C to 80°C.
I'm not sure what the notation "3:1" implies; perhaps that it's an impure mixture of reactive red 3 with a related molecule that gives the dye a slightly different hue. Could it be that the mixture contains some reactive red 1, given that it is described as being the chemical precursor for synthesis of reactive red 3? No, that is unlikely, since it is a dichlorotriazine dye; a mixture of two dyes of such different reactivity would be more difficult to work with.
(Please help support this web site. Thank you.)
Message: i required to know what is the chemical structure of reactive red 3:1 as i am not able to get the latest color index database. so, request you to sir, please if you have than please give me.
I don't have ready access to the Colour Index, either. Have you tried a web search? It's easy to find the CAS number for this dye, which is 23211-47-4, and with that you can find a great deal of additional information. Brand names under which "reactive red 3:1" or "reactive red 3" are sold appear to include Tulactiv Red P4BN and Brilliant Red K-2BP. The molecular formula is C25H15ClN7Na3O10S3, and the full chemical name is 2,7-Naphthalenedisulfonic acid, 5-((4-chloro-6-(phenylamino) -1,3,5-triazin-2-yl)amino)-4- hydroxy-3-((2-sulfophenyl)azo)-, trisodium salt.
The chemical structure for Colour Index reactive red 3 is listed online in US patent 4016149 as follows:

This structure is also given on page 190 of John Shore's excellent book, Cellulosics Dyeing, which is available for the very reasonable price of £9 when ordered directly from the Society of Dyers and Colourists in the UK. This dye is a 2-amino-4-chloro derivative of reactive red 1 (Procion Red MX-2B); the single chlorine makes it much less reactive, so it requires fixation temperatures of 70°C to 80°C.
I'm not sure what the notation "3:1" implies; perhaps that it's an impure mixture of reactive red 3 with a related molecule that gives the dye a slightly different hue. Could it be that the mixture contains some reactive red 1, given that it is described as being the chemical precursor for synthesis of reactive red 3? No, that is unlikely, since it is a dichlorotriazine dye; a mixture of two dyes of such different reactivity would be more difficult to work with.
(Please help support this web site. Thank you.)
Monday, September 10, 2007
I am interested in dying some tagua nut slices to use as jewelry. I want something that is safe to wear next to the skin...nothing toxic!
Name: kim
Message: hello!!
I am interested in dying some tagua nut slices to use as jewelry. I want something that is safe to wear next to the skin...nothing toxic!!! Do you have any suggestions or ideas to help me? Also, once the pieces are colored/dyed, is there anything safe that you might suggest to put over the nut slices to add a shine??? Again, it must be something that's safe.
How safe do your dyes need to be? Do they need to be food-safe? If so, the best dyes to use would be natural or synthetic food colorings, which have been tested and found to be safe for use in food. However, tagua nuts are said to be composed primarily of cellulose, and most food coloring dyes do not bond permanently to cellulose.
If the nuts are going to be worn against the skin, but not chewed on or sucked on (as a baby might do), then there is no need for the dyes to be food-safe. In this case, fiber reactive dyes such as Procion MX or Remazol dyes are safe. The dye is applied at a high pH, using soda ash, and then all excess unattached dye is washed out. Since Procion MX dyes bond well to cellulose under these conditions, they should be suitable for dyeing tagua nuts. Although careless use of fiber reactive dye powders can produce allergies in the dyer, the dye is completely safe for wear once it has bound to cellulose. After fiber reactive dyes have been properly applied and all excess dye washed out, they appear to be less likely to produce allergies in the wearer than other types of dye (such as direct dyes, basic dyes, or disperse dyes), judging from the reports indexed in MedLine, apparently because of the very strong bond between the fiber reactive dye and the cellulose molecule. Both Procion and Remazol dyes have been certified as safe for use in baby products in the Oeko-Tex Standard 100 list [PDF]; however, like most dyes, they have not been approved for safe use in foods.
Earthhues says that you can use their natural dyes to dye tagua nuts, after first mordanting with aluminum acetate, using a weight of aluminum acetate that is equal to 4% of the weight of the nuts. Madder is a natural red dye that works well on tagua nuts, but madder is not safe for food use (it may be carcinogenic when eaten); it is safe to wear, but not to eat. Cochineal, however, is a good dye, whether used on cellulose or protein fibers, and is commonly used in foodstuffs, including red yogurt and other red food items. Cochineal is made from the ground-up bodies of a particular type of cactus-eating insects. It will not work well on cellulose unless you use a mordant such as alum. Beware of mordants other than alum, as they can be quite toxic. Some people prefer to avoid all aluminum-containing materials (including alum) out of the mistaken fear that they cause Alzheimer's disease, which they do not.
There are two food items that can be used to dye cellulose without a mordant, which are turmeric and walnut husks. Turmeric is a spice, ground from roots of the turmeric plant, which dyes a bright yellow. It will fade from light exposure, and must be reapplied after some use, perhaps yearly. Walnut husks can be used to produce a deep brown. If you decide to use natural dyes, I recommend that you get a good book on the subject. Natural dyes are considerably more of a challenge to use than synthetic dyes, so you will need a good recipe and a good understanding of how to use mordants.
As far as producing a shine on the nuts, again, the question is whether the substance you use needs to be safe to be worn against the skin, or whether it has to be safe even when chewed or sucked on. In the latter case, it seems that you must stick to food-safe coatings. If you polish the nuts very well, using fine emory cloth or even a fingernail buffer, they will probably hold a shine. Shellac, a substance made from a kind of insect called the lac beetle, is commonly used in food and is available in food-grade quality; do not use non-food-grade shellac if you want your product to be safe to be put in the mouths of children. Beeswax can also be used to polish and produce a shine; it is easily obtained, is food-safe, and has a wonderful reputation as well for finishing natural wood baby toys. The solid paraffin wax sold in grocery stores for use in jelly-making is refined to the point of being commonly considered as food-safe, but has not been approved by the FDA as a food additive, in spite of generations of use (or misuse) as such.
(Please help support this web site. Thank you.)
Message: hello!!
I am interested in dying some tagua nut slices to use as jewelry. I want something that is safe to wear next to the skin...nothing toxic!!! Do you have any suggestions or ideas to help me? Also, once the pieces are colored/dyed, is there anything safe that you might suggest to put over the nut slices to add a shine??? Again, it must be something that's safe.
How safe do your dyes need to be? Do they need to be food-safe? If so, the best dyes to use would be natural or synthetic food colorings, which have been tested and found to be safe for use in food. However, tagua nuts are said to be composed primarily of cellulose, and most food coloring dyes do not bond permanently to cellulose.
If the nuts are going to be worn against the skin, but not chewed on or sucked on (as a baby might do), then there is no need for the dyes to be food-safe. In this case, fiber reactive dyes such as Procion MX or Remazol dyes are safe. The dye is applied at a high pH, using soda ash, and then all excess unattached dye is washed out. Since Procion MX dyes bond well to cellulose under these conditions, they should be suitable for dyeing tagua nuts. Although careless use of fiber reactive dye powders can produce allergies in the dyer, the dye is completely safe for wear once it has bound to cellulose. After fiber reactive dyes have been properly applied and all excess dye washed out, they appear to be less likely to produce allergies in the wearer than other types of dye (such as direct dyes, basic dyes, or disperse dyes), judging from the reports indexed in MedLine, apparently because of the very strong bond between the fiber reactive dye and the cellulose molecule. Both Procion and Remazol dyes have been certified as safe for use in baby products in the Oeko-Tex Standard 100 list [PDF]; however, like most dyes, they have not been approved for safe use in foods.
Earthhues says that you can use their natural dyes to dye tagua nuts, after first mordanting with aluminum acetate, using a weight of aluminum acetate that is equal to 4% of the weight of the nuts. Madder is a natural red dye that works well on tagua nuts, but madder is not safe for food use (it may be carcinogenic when eaten); it is safe to wear, but not to eat. Cochineal, however, is a good dye, whether used on cellulose or protein fibers, and is commonly used in foodstuffs, including red yogurt and other red food items. Cochineal is made from the ground-up bodies of a particular type of cactus-eating insects. It will not work well on cellulose unless you use a mordant such as alum. Beware of mordants other than alum, as they can be quite toxic. Some people prefer to avoid all aluminum-containing materials (including alum) out of the mistaken fear that they cause Alzheimer's disease, which they do not.
There are two food items that can be used to dye cellulose without a mordant, which are turmeric and walnut husks. Turmeric is a spice, ground from roots of the turmeric plant, which dyes a bright yellow. It will fade from light exposure, and must be reapplied after some use, perhaps yearly. Walnut husks can be used to produce a deep brown. If you decide to use natural dyes, I recommend that you get a good book on the subject. Natural dyes are considerably more of a challenge to use than synthetic dyes, so you will need a good recipe and a good understanding of how to use mordants.
As far as producing a shine on the nuts, again, the question is whether the substance you use needs to be safe to be worn against the skin, or whether it has to be safe even when chewed or sucked on. In the latter case, it seems that you must stick to food-safe coatings. If you polish the nuts very well, using fine emory cloth or even a fingernail buffer, they will probably hold a shine. Shellac, a substance made from a kind of insect called the lac beetle, is commonly used in food and is available in food-grade quality; do not use non-food-grade shellac if you want your product to be safe to be put in the mouths of children. Beeswax can also be used to polish and produce a shine; it is easily obtained, is food-safe, and has a wonderful reputation as well for finishing natural wood baby toys. The solid paraffin wax sold in grocery stores for use in jelly-making is refined to the point of being commonly considered as food-safe, but has not been approved by the FDA as a food additive, in spite of generations of use (or misuse) as such.
(Please help support this web site. Thank you.)
Sunday, September 09, 2007
could you explain to me what "exhaust" the dye means? Am I to understand the dye that has been mixed with vinegar becomes exhausted and then has little impact on the environment when I dump it out?
Name: blue
Message: Hi Paula,
I dye wool with mx reactive dye (soaking vinegar and applying dye), in this case could you explain to me what "exhaust" the dye means? Am I to understand the dye that has been mixed with vinegar becomes exhausted and then has little impact on the environment when I dump it out? What about the dye that is not mixed with vinegar but applied to wool that is vinegar soaked, I mean the squeeze bottle of dye, will time exhaust that so I can feel comfortable dumping it out?
Hope that makes sense. Totally understand if you can't reply.

It is safe to dispose of Procion MX dyes down the drain. In home-use quantities, it has little or no impact on the environment.
When dye "exhausts", that means only that it has mostly left the dyebath and become loosely associated with the fiber that you are dyeing. The next step is the formation of a chemical bond, which in the case of acid dyes is most likely a hydrogen bond.
Dyeing wool is a fairly neat process, because in many cases all of the dye leaves the solution and exhausts onto the fabric, resulting in essentially colorless water. Dyeing cotton is a very different experience, because there is always a lot of dye remaining in the water after dyeing. However, for individual dyers or small studios, the quantities used are not a problem.
Disposing of household-use quantities of reactive dyes in the public sewer or a septic system is legal and is unlikely to have any impact on the environment; it's a very different situation from the vast quantities of effluant produced by a textile mill. The dyes we use are not particularly toxic. Disposing of Procion MX dyes will not damage your septic system. Procion MX dyes are either metal-free, or, in the case of some colors, contain up to 2% to 5% of a metal ion such as copper (not chromium). In the quantities that you are likely to be using, unless you have a large business, this is insignificant. If you have a septic system, it is sometimes recommended that you neutralize strongly acid solutions with baking soda, or strongly basic solutions with vinegar, before pouring them down the drain, because extremes of pH can be bad for the bacteria that are needed for the proper functioning of a septic tank. However, the dilution of the dyebath by other water that you put down the drain, in the course of a normal day, will probably be sufficient to bring the pH to an adequately neutral level.
Even if you use a chromium-containing premetalized acid dye, such as Lanaset Jet Black, the overall concentration of the chromium in the dyebath is low enough to present no problem for disposal. (As I calculated on an earlier occasion, a dye painting solution of 1 teaspoon of Jet Black Lanaset dye that contains 2.5 grams of dye, dissolved in one cup (250 ml) of water, contains 0.08 grams of chromium; after being diluted with 50 gallons of uncontaminated water, this dye concentration would meet the US EPA standard for chromium content of drinking water in the US, which is 100 micrograms per liter.) If you set up a small factory to use large quantities of dye, the situation will be different. In that case, you will have to find out the local regulations for dye disposal, but this is not an issue for a single dye artist.
Some mordants are extremely toxic. For example, while the chromium contained in a metal complex dye like Lanaset Jet Black is in the relatively safe trivalent form, the chromium in potassium dichromate solution, or that used with another class of wool dyes called chrome dyes, is the much more hazardous hexavalent form of chromium, listed by the EPA as a human carcinogen. I would not advise the use of chromium mordant solutions at home or in an art studio. They are quite different from working with safer dyes.
More information about disposing of Procion Dyes:
http://www.dharmatrading.com/info/procion_general_info.html
http://www.prochemical.com/StudioSafety.htm
Some other kinds of dyes should not be disposed of down the sink. For example, Pebeo Soie silk dye should be disposed of the same way as unused housepaint, which should not be put down the drain or put in the regular trash. Check the "Waste Disposal" section of the MSDS for the specific dye you are using. Your dye supplier will give you an MSDS for your dye if you request it.
(Please help support this web site. Thank you.)
Message: Hi Paula,
I dye wool with mx reactive dye (soaking vinegar and applying dye), in this case could you explain to me what "exhaust" the dye means? Am I to understand the dye that has been mixed with vinegar becomes exhausted and then has little impact on the environment when I dump it out? What about the dye that is not mixed with vinegar but applied to wool that is vinegar soaked, I mean the squeeze bottle of dye, will time exhaust that so I can feel comfortable dumping it out?
Hope that makes sense. Totally understand if you can't reply.
Jacquard Acid Dyes are concentrated, powdered, hot water dyes that produce the most vibrant possible results on protein fibers including mohair, silk, wool, cashmere, alpaca, feathers, and most nylons.
It is safe to dispose of Procion MX dyes down the drain. In home-use quantities, it has little or no impact on the environment.
When dye "exhausts", that means only that it has mostly left the dyebath and become loosely associated with the fiber that you are dyeing. The next step is the formation of a chemical bond, which in the case of acid dyes is most likely a hydrogen bond.
Dyeing wool is a fairly neat process, because in many cases all of the dye leaves the solution and exhausts onto the fabric, resulting in essentially colorless water. Dyeing cotton is a very different experience, because there is always a lot of dye remaining in the water after dyeing. However, for individual dyers or small studios, the quantities used are not a problem.
Disposing of household-use quantities of reactive dyes in the public sewer or a septic system is legal and is unlikely to have any impact on the environment; it's a very different situation from the vast quantities of effluant produced by a textile mill. The dyes we use are not particularly toxic. Disposing of Procion MX dyes will not damage your septic system. Procion MX dyes are either metal-free, or, in the case of some colors, contain up to 2% to 5% of a metal ion such as copper (not chromium). In the quantities that you are likely to be using, unless you have a large business, this is insignificant. If you have a septic system, it is sometimes recommended that you neutralize strongly acid solutions with baking soda, or strongly basic solutions with vinegar, before pouring them down the drain, because extremes of pH can be bad for the bacteria that are needed for the proper functioning of a septic tank. However, the dilution of the dyebath by other water that you put down the drain, in the course of a normal day, will probably be sufficient to bring the pH to an adequately neutral level.
Even if you use a chromium-containing premetalized acid dye, such as Lanaset Jet Black, the overall concentration of the chromium in the dyebath is low enough to present no problem for disposal. (As I calculated on an earlier occasion, a dye painting solution of 1 teaspoon of Jet Black Lanaset dye that contains 2.5 grams of dye, dissolved in one cup (250 ml) of water, contains 0.08 grams of chromium; after being diluted with 50 gallons of uncontaminated water, this dye concentration would meet the US EPA standard for chromium content of drinking water in the US, which is 100 micrograms per liter.) If you set up a small factory to use large quantities of dye, the situation will be different. In that case, you will have to find out the local regulations for dye disposal, but this is not an issue for a single dye artist.
Some mordants are extremely toxic. For example, while the chromium contained in a metal complex dye like Lanaset Jet Black is in the relatively safe trivalent form, the chromium in potassium dichromate solution, or that used with another class of wool dyes called chrome dyes, is the much more hazardous hexavalent form of chromium, listed by the EPA as a human carcinogen. I would not advise the use of chromium mordant solutions at home or in an art studio. They are quite different from working with safer dyes.
More information about disposing of Procion Dyes:
http://www.dharmatrading.com/info/procion_general_info.html
http://www.prochemical.com/StudioSafety.htm
Some other kinds of dyes should not be disposed of down the sink. For example, Pebeo Soie silk dye should be disposed of the same way as unused housepaint, which should not be put down the drain or put in the regular trash. Check the "Waste Disposal" section of the MSDS for the specific dye you are using. Your dye supplier will give you an MSDS for your dye if you request it.
(Please help support this web site. Thank you.)
Saturday, September 08, 2007
using different dirts from all over the state to color a quilt
Name: Debbie
Message: I am working on a project for our state's Centenial. Another teacher that I work with does a project using dirt from all over the state. I would like to use the different dirt to dye fabric for a quilt. The fabric needs to be cotton but it will probably not be washed often or ever but I would still like for it to be somewhat colorfast. Any suggestions? Do I need to know the makeup of the dirt to determine the best way to dye the fabric?
Dyeing cotton with dirt tends to result in much blander, paler colors than the color of the dirt, since most of the dirt tends to wash out. Your quilt might not have a problem, though, since it may be able to escape being washed.
Most dirt is colored with iron. The iron in red dirt is hematite, while that in yellow dirt is limonite. The ultimate color obtained by dyeing cotton with iron is, typically, a buff color. The color obtained by using dirt as a true dye will be less exciting than that which you could obtain by using it as a pigment in a homemade fabric paint, since using it as a pigment does not require the iron to bind to the fiber using its own chemical properties.
The method that I have been thinking about, but which I still have not tried, is to buy some colorless fabric paint binder, such as Neopaque clear Flowable
Extender (you can order this stuff from MisterArt.com or from Dharma Trading Company). Mixing different dirts with this clear fabric paint binder will turn it into a fabric paint. It will take a little trial and error to see how much dirt to add to a given volume of colorless binder. Depending on the thickness with which you apply your fabric paint, you may be able to cover anywhere from 4 to 15 square feet with one 250 ml jar of your paint. The thinner application will have a softer feel, but less intense colors. The paint will produce a more dye-like effect when applied to wet fabric. Fabric paint binder should be easier than other methods you might use to color your cotton with dirt. You will need to heat-set the fabric with an iron, after the paint has dried, to melt the binders that will permanently attach your dirt pigments to the fabric; follow the heat-setting instructions provided by the paint extender's manufacturer. One method you could use for applying the paint would be to dilute the paint with a little water, soak small pieces of fabric in it, and then squeeze the fabric out before allowing it to dry. Another is to stretch the fabric out, moisten it with a spray bottle of water, and apply the paint with a brush.

A more traditional method, depending on your traditions, is the Japanese method of using freshly homemade soy milk as a binder to "glue" your dirt pigment to the fabric. This will be quite a bit more trouble and will not stand up to modern washing methods, not that that's likely to be a problem for your quilt. You can find instructions for this method, and links to buy materials, at Table Rock Llamas.
Before using either of the above methods to prepare your own fabric paint, you should crush and sift your dirt samples to make sure that the size of the particles is small and uniform. Larger chunks of dirt will have more difficulty in adhering to the fabric, regardless of the convenience of the fabric paint binder. The smallest particle size you can obtain will be best. I would advise the use of a mortar and pestle to crush lumps of thoroughly dried dirt.
I would very much like to learn about your experiences in making this quilt.
(Please help support this web site. Thank you.)
Message: I am working on a project for our state's Centenial. Another teacher that I work with does a project using dirt from all over the state. I would like to use the different dirt to dye fabric for a quilt. The fabric needs to be cotton but it will probably not be washed often or ever but I would still like for it to be somewhat colorfast. Any suggestions? Do I need to know the makeup of the dirt to determine the best way to dye the fabric?
Dyeing cotton with dirt tends to result in much blander, paler colors than the color of the dirt, since most of the dirt tends to wash out. Your quilt might not have a problem, though, since it may be able to escape being washed.
Most dirt is colored with iron. The iron in red dirt is hematite, while that in yellow dirt is limonite. The ultimate color obtained by dyeing cotton with iron is, typically, a buff color. The color obtained by using dirt as a true dye will be less exciting than that which you could obtain by using it as a pigment in a homemade fabric paint, since using it as a pigment does not require the iron to bind to the fiber using its own chemical properties.
The method that I have been thinking about, but which I still have not tried, is to buy some colorless fabric paint binder, such as Neopaque clear Flowable

Extender (you can order this stuff from MisterArt.com or from Dharma Trading Company). Mixing different dirts with this clear fabric paint binder will turn it into a fabric paint. It will take a little trial and error to see how much dirt to add to a given volume of colorless binder. Depending on the thickness with which you apply your fabric paint, you may be able to cover anywhere from 4 to 15 square feet with one 250 ml jar of your paint. The thinner application will have a softer feel, but less intense colors. The paint will produce a more dye-like effect when applied to wet fabric. Fabric paint binder should be easier than other methods you might use to color your cotton with dirt. You will need to heat-set the fabric with an iron, after the paint has dried, to melt the binders that will permanently attach your dirt pigments to the fabric; follow the heat-setting instructions provided by the paint extender's manufacturer. One method you could use for applying the paint would be to dilute the paint with a little water, soak small pieces of fabric in it, and then squeeze the fabric out before allowing it to dry. Another is to stretch the fabric out, moisten it with a spray bottle of water, and apply the paint with a brush.
A more traditional method, depending on your traditions, is the Japanese method of using freshly homemade soy milk as a binder to "glue" your dirt pigment to the fabric. This will be quite a bit more trouble and will not stand up to modern washing methods, not that that's likely to be a problem for your quilt. You can find instructions for this method, and links to buy materials, at Table Rock Llamas.
Before using either of the above methods to prepare your own fabric paint, you should crush and sift your dirt samples to make sure that the size of the particles is small and uniform. Larger chunks of dirt will have more difficulty in adhering to the fabric, regardless of the convenience of the fabric paint binder. The smallest particle size you can obtain will be best. I would advise the use of a mortar and pestle to crush lumps of thoroughly dried dirt.
I would very much like to learn about your experiences in making this quilt.
(Please help support this web site. Thank you.)
Friday, September 07, 2007
my twin sons want to tie dye shirts at the party as a party favor
Name: Jen
Message: Hi - I am having a party in a few weeks for my twin 6 (almost 7) year old sons. We are having this party in a park. They want to tie dye shirts at the party as a party favor. (Only one color per shirt - I really don't want to deal with the squirt bottles!) I figured I could just get some huge plastic buckets full of warm water and bring it to the park - then I'll have the kids tie and I will dye. What I am wondering is what steps can I do before hand to help move the process along? Would it make more sense to dump them in the tub and let them soak?? Or could I dip them briefly and then bag them up and send them home?? I know I want a cold dye and I'm wondering if it would make it easier to use the kind that already has the soda ash mix in so I don't need to soak them before hand, and then, I am assuming, the kids wouldn't need to wear gloves while they were tying, correct? (Since there would be no soda ash on the shirts before hand and I will be the one doing the dyeing.) anyway, I have read a bunch of the questions and answers on your site (MOST helpful) but I was hoping I could pick your brain a little more specifically - Thanks,Jen
The dye you want to use would definitely be a cool water fiber reactive dye, such as Procion MX dye. This dye does not require any heat setting at all, though warmer temperatures work more quickly. Don't use cold water; use water that is at least 70°F, or a little warmer if possible. (Don't use an all-purpose dye such as Rit or Tintex, as they are hot water dyes.)
I would not want to have the shirts presoaked in soda ash for the kids to tie. I think it is best for them to tie dry shirts. I always tie my shirts either dry or moistened with plain water, then drop them in a bucket of soda ash to presoak. You can do the same, or you can add the soda ash directly to the dye (or use the kits that have soda ash already added to the dye) immediately before use.
If you are using dye that does not have soda ash mixed in already, I would advise you to mix the dye with water in advance. It would be easier to carry quart jars of dye concentrate, something with a good screw-on lid, than to carry large volumes of dye in a bucket, but it takes a little bit of trouble to get the dye dissolved, something you don't need to be messing with at a party. You can mix the dye with cool or room-temperature dye up to one week in advance, if it does not have soda ash in it. Keep the dye at room temperature or colder until you are ready to use it. Refrigeration is fine. If you use dye that is premixed with soda ash, DO NOT mix the dye in advance. Dye + water + soda ash = immediate dye reaction. Once the dye has been mixed with soda ash and water, it will be used up quickly.
You will need soda ash to set the dye. This is fairly easy to dissolve in water, so you don't necessarily have to predissolve it before the party. Do not add the soda ash to the dye until you are ready to add the shirts, or even after you have put the shirts in, because the dye will be active for probably no more than one hour after you add the fabric.
Salt is optional; it is normally required for immersion dyeing, but you could use just enough dye and water to thoroughly soak the tied shirts, rather than a large excess of water as is normally used for immersion dyeing. The large amount of water is needed only to get smooth, even, solid colors, obviously not particularly desirable for this project. Using smaller amounts of water, as in low water immersion dyeing, will be a lot less trouble to deal with.
Dipping the shirts will work, as long as you make sure the dye thoroughly soaks the shirts, which will not take long. Or you could put each shirt in its bag and dump in some dye solution and soda ash solution. You won't need urea, though it won;t hurt anything, because the plastic bags can substitute for urea's function of keeping the dye moist long enough for the reaction to complete.
The squirt bottle thing can be made more manageable for multiple colors if you want to have an adult add the dye. When I did a dye demonstration for my son's fifth grade class, I had three bottles of dye (turquoise, fuchsia, and lemon yellow) and asked each child which two colors he or she wanted. They each had a small cotton handkerchief in a ziplock sandwich bag, to which I'd already added soda ash in water. You could put a t-shirt into a gallon-sized ziplock bag. Note that the bags labeled for freezer use are sturdier and less likely to leak. I did not give the children the choice of using three colors, because all three colors will make a muddy brown color when combined together. Any two primary colors mix well, though (yellow plus turquoise makes green, yellow plus fuchsia makes orange and red, and turquoise plus fuchsia makes purple). It would be more fun to use the same two colors for everyone's shirt than to use only one color, and there's not much mess if an adult does the actual squirting.
(Please help support this web site. Thank you.)
Message: Hi - I am having a party in a few weeks for my twin 6 (almost 7) year old sons. We are having this party in a park. They want to tie dye shirts at the party as a party favor. (Only one color per shirt - I really don't want to deal with the squirt bottles!) I figured I could just get some huge plastic buckets full of warm water and bring it to the park - then I'll have the kids tie and I will dye. What I am wondering is what steps can I do before hand to help move the process along? Would it make more sense to dump them in the tub and let them soak?? Or could I dip them briefly and then bag them up and send them home?? I know I want a cold dye and I'm wondering if it would make it easier to use the kind that already has the soda ash mix in so I don't need to soak them before hand, and then, I am assuming, the kids wouldn't need to wear gloves while they were tying, correct? (Since there would be no soda ash on the shirts before hand and I will be the one doing the dyeing.) anyway, I have read a bunch of the questions and answers on your site (MOST helpful) but I was hoping I could pick your brain a little more specifically - Thanks,Jen
The dye you want to use would definitely be a cool water fiber reactive dye, such as Procion MX dye. This dye does not require any heat setting at all, though warmer temperatures work more quickly. Don't use cold water; use water that is at least 70°F, or a little warmer if possible. (Don't use an all-purpose dye such as Rit or Tintex, as they are hot water dyes.)
I would not want to have the shirts presoaked in soda ash for the kids to tie. I think it is best for them to tie dry shirts. I always tie my shirts either dry or moistened with plain water, then drop them in a bucket of soda ash to presoak. You can do the same, or you can add the soda ash directly to the dye (or use the kits that have soda ash already added to the dye) immediately before use.
If you are using dye that does not have soda ash mixed in already, I would advise you to mix the dye with water in advance. It would be easier to carry quart jars of dye concentrate, something with a good screw-on lid, than to carry large volumes of dye in a bucket, but it takes a little bit of trouble to get the dye dissolved, something you don't need to be messing with at a party. You can mix the dye with cool or room-temperature dye up to one week in advance, if it does not have soda ash in it. Keep the dye at room temperature or colder until you are ready to use it. Refrigeration is fine. If you use dye that is premixed with soda ash, DO NOT mix the dye in advance. Dye + water + soda ash = immediate dye reaction. Once the dye has been mixed with soda ash and water, it will be used up quickly.
You will need soda ash to set the dye. This is fairly easy to dissolve in water, so you don't necessarily have to predissolve it before the party. Do not add the soda ash to the dye until you are ready to add the shirts, or even after you have put the shirts in, because the dye will be active for probably no more than one hour after you add the fabric.
Salt is optional; it is normally required for immersion dyeing, but you could use just enough dye and water to thoroughly soak the tied shirts, rather than a large excess of water as is normally used for immersion dyeing. The large amount of water is needed only to get smooth, even, solid colors, obviously not particularly desirable for this project. Using smaller amounts of water, as in low water immersion dyeing, will be a lot less trouble to deal with.
Dipping the shirts will work, as long as you make sure the dye thoroughly soaks the shirts, which will not take long. Or you could put each shirt in its bag and dump in some dye solution and soda ash solution. You won't need urea, though it won;t hurt anything, because the plastic bags can substitute for urea's function of keeping the dye moist long enough for the reaction to complete.
The squirt bottle thing can be made more manageable for multiple colors if you want to have an adult add the dye. When I did a dye demonstration for my son's fifth grade class, I had three bottles of dye (turquoise, fuchsia, and lemon yellow) and asked each child which two colors he or she wanted. They each had a small cotton handkerchief in a ziplock sandwich bag, to which I'd already added soda ash in water. You could put a t-shirt into a gallon-sized ziplock bag. Note that the bags labeled for freezer use are sturdier and less likely to leak. I did not give the children the choice of using three colors, because all three colors will make a muddy brown color when combined together. Any two primary colors mix well, though (yellow plus turquoise makes green, yellow plus fuchsia makes orange and red, and turquoise plus fuchsia makes purple). It would be more fun to use the same two colors for everyone's shirt than to use only one color, and there's not much mess if an adult does the actual squirting.
(Please help support this web site. Thank you.)
Thursday, September 06, 2007
How can I remove the bleach I used to clean mold off of an upholstered chair?
Name: Michal
Message: Hi, can you help please! I have an upholstered lounge chair which was covered in thick black mold as it had been left outdoors in the damp. I tried to clean it off with a water & bleach solution but found it didn't budge so eventually I had to use straight bleach(Clorox) which I rubbed all over...it worked and the chair is like new..except when you sit on it with damp clothes as the bleach is still active in the upholstery (which wasn't damaged). How can I get the bleach out completely? The bleach is probably in the wadding too. If I used Anti-chlor how would I do it? Would a steam & dry vacume clean be a good idea to finish off with? Thank you for any advice you might have.
This is an interesting question. I'm surprised at how well the bleach worked! Often a bad mold growth like that is pretty much impossible to remove. The upholstery must be made of very sturdy fabric. You might find that it will show wear quickly, though, after the damage it's probably sustained.
The most important thing, now that you have treated it with bleach, is that you must rinse the chair. I believe that there is no way around this. You should take it outside and hose it off, or dump buckets of water over it. Water is not good for upholstery, but toxic chemicals such as chlorine bleach are worse. It may be best to remove the upholstery altogether and reupholster the chair.
After you've rinsed the chair, I think it would be best to soak that chair with Anti-chlor just as you earlier did with bleach. There are different bleach stopping chemicals you can use, but you are going to have to use a large amount, and Anti-chlor (sodium bisulfite) is the most economical. (See "How can I neutralize the damaging effects of chlorine bleach?".)
Apply the Anti-chlor outside or with large fans providing excellent ventilation from open windows and doors. It's less toxic and less dangerous than chlorine bleach, but it can cause respiratory irritation, especially in people who have asthma. Read and follow the MSDS (safety precautions page) supplied by the company from which you buy it.
You can mail order Anti-chlor, that is, sodium bisulfite, from PRO Chemical & Dye or another supplier, or purchase sodium bisulfite, potassium bisulfite, sodium metabisulfite, or potassium metabisulfite from a winemaking supply store. Dissolve it in water, using one teaspoon per 2.5 gallons of water. Throughly soak the chair with it, let it sit for fifteen minutes or more, and then rinse out by pouring many bucketsful of water over the chair.
Hydrogen peroxide, the 3% strength used as an antiseptic, is a safe alternative to Anti-chlor, but it will cost more to buy a large enough quantity to soak the chair. You need to use plenty, to make sure that you have gotten enough into every place that the bleach got into.
Although steam cleaning afterwards, as you suggest, may be a good idea, I don't believe that it can substitute for rinsing with large quantities of water.
(Please help support this web site. Thank you.)
Message: Hi, can you help please! I have an upholstered lounge chair which was covered in thick black mold as it had been left outdoors in the damp. I tried to clean it off with a water & bleach solution but found it didn't budge so eventually I had to use straight bleach(Clorox) which I rubbed all over...it worked and the chair is like new..except when you sit on it with damp clothes as the bleach is still active in the upholstery (which wasn't damaged). How can I get the bleach out completely? The bleach is probably in the wadding too. If I used Anti-chlor how would I do it? Would a steam & dry vacume clean be a good idea to finish off with? Thank you for any advice you might have.
This is an interesting question. I'm surprised at how well the bleach worked! Often a bad mold growth like that is pretty much impossible to remove. The upholstery must be made of very sturdy fabric. You might find that it will show wear quickly, though, after the damage it's probably sustained.
The most important thing, now that you have treated it with bleach, is that you must rinse the chair. I believe that there is no way around this. You should take it outside and hose it off, or dump buckets of water over it. Water is not good for upholstery, but toxic chemicals such as chlorine bleach are worse. It may be best to remove the upholstery altogether and reupholster the chair.
After you've rinsed the chair, I think it would be best to soak that chair with Anti-chlor just as you earlier did with bleach. There are different bleach stopping chemicals you can use, but you are going to have to use a large amount, and Anti-chlor (sodium bisulfite) is the most economical. (See "How can I neutralize the damaging effects of chlorine bleach?".)
Apply the Anti-chlor outside or with large fans providing excellent ventilation from open windows and doors. It's less toxic and less dangerous than chlorine bleach, but it can cause respiratory irritation, especially in people who have asthma. Read and follow the MSDS (safety precautions page) supplied by the company from which you buy it.
You can mail order Anti-chlor, that is, sodium bisulfite, from PRO Chemical & Dye or another supplier, or purchase sodium bisulfite, potassium bisulfite, sodium metabisulfite, or potassium metabisulfite from a winemaking supply store. Dissolve it in water, using one teaspoon per 2.5 gallons of water. Throughly soak the chair with it, let it sit for fifteen minutes or more, and then rinse out by pouring many bucketsful of water over the chair.
Hydrogen peroxide, the 3% strength used as an antiseptic, is a safe alternative to Anti-chlor, but it will cost more to buy a large enough quantity to soak the chair. You need to use plenty, to make sure that you have gotten enough into every place that the bleach got into.
Although steam cleaning afterwards, as you suggest, may be a good idea, I don't believe that it can substitute for rinsing with large quantities of water.
(Please help support this web site. Thank you.)
Tuesday, September 04, 2007
I recently bought a cream dress made from 100% Polyester. I wore it the once to a party but when I got home I noticed a huge stain on the back which i can't seem to wash out.
Name: Kelly
Message: Hi, I hope you can help me.
I recently bought a cream dress made from 100% Polyester. I wore it the once to a party but when I got home I noticed a huge stain on the back which i can't seem to wash out. I'm not sure what it is but it is like a yellow colour. I don't want to throw the dress away as it cost me a fortune & I really like it. Can you tell me if it is possible to dye the dress...I don't mind it if have to change the colour to black if it makes it easier to hide the stain?? If the dress can be dyed will it look like it's been dyed? Is it likely to have patches of darker colour is certain areas? Can I find anyone to do it professionally in case i make a mistake?? Please help....I'm really scared about ruining the dress anymore.
I'm sorry, but dyeing a polyester dress would require boiling it for an hour with a special kind of dye called disperse dye, plus a toxic carrier chemical. The pot you use to boil it in cannot be a cheap one, because it must be non-aluminum and large enough to allow the dress to move freely throughout the boiling process (to avoid having some regions darker and some lighter), and yet it should never be used for food again afterwards, because textile dyes are not considered safe for use in food containers. The cost of the pot might exceed that of another dress.
There is also the question of whether or not the dress would survive such harsh treatment without problems with the stitching or the trim. There is no dyeing service that will be happy to try to dye a 100% polyester garment. For more information please read: "Dyeing Polyester with Disperse Dyes".
A third problem is that, as dye is transparent, stains will continue to show up as a different color even after dyeing; for more on this topic, see "How can I fix the bleach spots on my favorite clothing?", which covers a similar problem.
I'm afraid that dyeing is not an answer to the problem of your stained polyester dress. It might still be possible to remove the stain, however. Avoid the use of chlorine bleach, as it can damage polyester badly, leaving a permanent yellow stain. Try using an oxygen-based colorsafe bleach, such as OxyClean or OxyBoost, or the 3% hydrogen peroxide sold as a disinfectant; also (not at the same time) try a reducing agent such as Rit Color Remover, which removes color much like bleach, but without damaging the polyester, when used according to the instructions.
(Please help support this web site. Thank you.)
Message: Hi, I hope you can help me.
I recently bought a cream dress made from 100% Polyester. I wore it the once to a party but when I got home I noticed a huge stain on the back which i can't seem to wash out. I'm not sure what it is but it is like a yellow colour. I don't want to throw the dress away as it cost me a fortune & I really like it. Can you tell me if it is possible to dye the dress...I don't mind it if have to change the colour to black if it makes it easier to hide the stain?? If the dress can be dyed will it look like it's been dyed? Is it likely to have patches of darker colour is certain areas? Can I find anyone to do it professionally in case i make a mistake?? Please help....I'm really scared about ruining the dress anymore.
I'm sorry, but dyeing a polyester dress would require boiling it for an hour with a special kind of dye called disperse dye, plus a toxic carrier chemical. The pot you use to boil it in cannot be a cheap one, because it must be non-aluminum and large enough to allow the dress to move freely throughout the boiling process (to avoid having some regions darker and some lighter), and yet it should never be used for food again afterwards, because textile dyes are not considered safe for use in food containers. The cost of the pot might exceed that of another dress.
There is also the question of whether or not the dress would survive such harsh treatment without problems with the stitching or the trim. There is no dyeing service that will be happy to try to dye a 100% polyester garment. For more information please read: "Dyeing Polyester with Disperse Dyes".
A third problem is that, as dye is transparent, stains will continue to show up as a different color even after dyeing; for more on this topic, see "How can I fix the bleach spots on my favorite clothing?", which covers a similar problem.
I'm afraid that dyeing is not an answer to the problem of your stained polyester dress. It might still be possible to remove the stain, however. Avoid the use of chlorine bleach, as it can damage polyester badly, leaving a permanent yellow stain. Try using an oxygen-based colorsafe bleach, such as OxyClean or OxyBoost, or the 3% hydrogen peroxide sold as a disinfectant; also (not at the same time) try a reducing agent such as Rit Color Remover, which removes color much like bleach, but without damaging the polyester, when used according to the instructions.
(Please help support this web site. Thank you.)
