|
The Chemistry of Dyeing: Reactive Dyes
a lesson
plan for beginning chemists from elementary through high school
written and prepared by Paula E. Burch, Ph.D.
(copyright 2003)
(To prepare for the most effective use of this lesson,
first construct models of the molecules pictured below,
using a Zome
building set.)
Everything we do, from digesting our food to making art,
involves chemistry. Everything is made of chemicals.
Some dyes, such as the kind you can buy in the grocery
store here in the US, really just stain clothes, so the dye washes out a
little every time you wash it. A really good dye actually
chemically attaches to the molecules of the fabric and can
never be washed out.
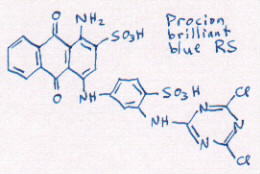 [bring out model of dye molecule blue MX-R]
[bring out model of dye molecule blue MX-R]
A molecule is much too tiny to see, but we can use models to
show what the dye molecule is shaped like. Each of these balls
represents a different sort of atom. These Cs are carbon
atoms, like you see in charcoal. The Os are oxygen, like in
the air you breathe, and these Cls are chlorine, like in
bleach. This model shows you what the blue molecules in this
bottle are shaped like. [note: in the
drawing, the C or Carbon atoms are represented by any corner
where two lines come together. All other atoms are spelled
out with their own one- or two-letter abbreviation, Cl for
Chlorine, O for Oxygen, etc.]
[show bottle of diluted blue MX-R]
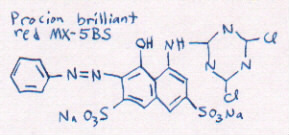 Different dye colors are made of different dye
molecules. Here is a model of another dye molecule.
Different dye colors are made of different dye
molecules. Here is a model of another dye molecule.
[hold up model of dye molecule red MX-8B]
The water containing that kind of dye molecule is red, as
you can see.
[show bottle of diluted red MX-8B]
You can see that the models are shaped a little
differently. Each different shape of dye molecule absorbs
light differently. That's what makes the different colors!
The fabric your clothing is made out of is also made of
molecules. Cotton, which grows on a cotton plant, is made of
long strands of cellulose molecules, all twisted
together. Cellulose is the same thing that wood is made
of. Here is a model of a cellulose molecule:
[model showing rope with -OH groups sticking out]
If you put these two molecules, the dye and the cotton,
together, nothing will happen, unless you can get some of
the atoms on the surfaces to come unstuck. If the H comes
off of the cellulose, and the Cl comes off of one end of the
dye molecule, the molecules will be able to react with each
other and stick together.
[wiggle -H of an -OH group on cellulose model, and
wiggle a -Cl on dye model]
How do we get the H and the Cl to get off of the cellulose
and the dye? We just add another chemical, called sodium
carbonate:
[model of Na2CO3]
What this does is increase the pH. That's how we say that it
makes it less acid. You already know about some acids -
vinegar and lemon juice are sour because they are acids. An
acid has a low pH. The opposite of acid is called a
base. You've probably seen a base in your kitchen, called
baking soda. When you put baking soda in water, you get a
high pH. You can taste the baking soda in your kitchen, but
don't taste these chemicals! A high pH is all that is
needed to get the dye and the cellulose ready to
react. Sodium carbonate is stronger than baking soda, so it
works better for dyeing.
[pull H off of cellulose model and Cl off of dye model]
[stick dye molecule model to cellulose model]
All we have to do to make a permanent bond between the dye
and the cotton is to put the dye on the cotton and add
washing soda. We can put the sodium carbonate on the fabric
before or after we put on the dye. After we put the dye and
the sodium carbonate on the fabric, we just have to wait a
while. While we wait, the reaction is happening - chlorines
are coming off the of the dye molecules and hydrogens are
coming off of the cellulose molecules. If they do this right
next to each other, the dye then attaches to the cellulose,
and a permanant bond is formed. If we leave it in a warm
room for a few hours, we can then wash the excess dye
out. We have to rinse it in cold water and wash it with
detergent in hot water to get all the extra dye off. After
all the excess dye is out, the dye left on the fabric is
permanant and looks like this.
[hold up dyed handkerchief]
[demonstrate dyeing as follows]
Materials:
- one handkerchief for each child, already soaked in soda ash,
wrung out, and placed in individual ziplock bags
- one bottle each of fuchsia and sky blue dissolved in water
Give each child a bag containing a slightly damp
handerchief. Ask them which color they want, and whether
they want one or two colors. Squirt small amount of dye into
each child's bag as requested. Make sure bag is locked
shut. Hand out another bag to double bag for increased
spill resistance.
[Hand out paper with today's lesson on it, including a
footnote on various places they can buy fiber reactive dyes
for use at home.]
|
