« 2007 August | Main | 2007 June »
Tuesday, July 31, 2007
I got bleach on my new area rug, what can I use to put the light grey color back?
Each grade is picking one color for their tie-dye shirt. I was wondering what process you thought would be the safest and easiest to use?
Monday, July 30, 2007
can I correct bleach damage on a spandex blend blouse?
Sunday, July 29, 2007
why do yellow candles burn faster than white, blue, or red candles?
Friday, July 27, 2007
I have a printed dress that I would like to dye a solid color. Must I remove the color first? and how??
Thursday, July 26, 2007
I made the mistake of buying my bridesmaid dresses on-line. They are a red burgundy and I was shooting for a deeper color, more of a wine or egg plant color. The fabric is polyester satin. What can I use to dye this?
Wednesday, July 25, 2007
I need to dye 100% olefin that has scotchgard on it.
Tuesday, July 24, 2007
I am trying to find Saxon Blue indigo liquid extract, as Trudy Van Stralen describes in her book Indigo Madder and Marigold. Do you use or know of a source for this liquid?
Monday, July 23, 2007
Is there any way to create a custom camouflage pattern with natural dyes (they give a better tone for my purposes), such that the pattern will neither wash out nor leak in the rain?
Sunday, July 22, 2007
My daughter and I are planning to dye 40 5.6 oz white cotton t-shirts. We are tie-dying them one color using RIT. How many boxes should we purchase?
Friday, July 20, 2007
Do I have to presoak it again in the soda ash when I re-dye?
Wednesday, July 18, 2007
dyeing a cotton slipcover in a front-loading washing machine
Tuesday, July 17, 2007
What is cold water dye and how is it used? I have Dylon cold dye and I want to use it.
Monday, July 16, 2007
I have an evening dress that is 100% polyester BUT it says handwash in cold water. Can it be dyed? It's baby blue.
Sunday, July 15, 2007
Is it possible to dye red Cordura nylon fabric to yellow?
Saturday, July 14, 2007
I have a lot of fifty to sixty year old linens that have turn yellow. Is there anyway that I can salvage them?
Friday, July 13, 2007
We are planning to tie dye a blanket for the horse but having problems coming up with a REALLY neat tie dyeing pattern so can you please help us out?
Thursday, July 12, 2007
My deep, intense colors came out pastel in the wash-out!
Wednesday, July 11, 2007
I already used all purpose dye - some of these were for children. Is that a serious problem?
Tuesday, July 10, 2007
I am trying to tie dye foldover elastic but the colors just wash away. So far I have used Procion MX and Rit dye products but neither have worked. What am I doing wrong?
Thursday, July 05, 2007
Can you please tell me if it's possible to dye black or gray clothing, & if so, how?
Wednesday, July 04, 2007
What is the purpose of salt in the dye mix?
Tuesday, July 03, 2007
I used to have a recipe that used resist salts L, calgon, and urea mixed with soda ash and bicarb. The results are super but I have lost the recipe do you have it?
Monday, July 02, 2007
What I am looking for is that amazing in-your-face clear blue that shows up in Nancy Crow's quilts. Any ideas?
Sunday, July 01, 2007
what dye would you recommend for the following garment: South American made jumpsuit with the following "espanol" materials - 60% algodao, 30% poliester, 10% elastano.
I got bleach on my new area rug, what can I use to put the light grey color back?
Name: marge
Message: I got bleach on my new area rug, what can I use to put the light grey color back?
There is a product that I have never tried that claims to redye bleach stains on carpets to their original color. See http://www.ecarpetstains.com/. I cannot vouch for how well it may work, since I have not used it myself. The advantage of this treatment is that it claims to be usable on just the bleach stain itself.
As a general rule, trying to dye an entire item to cover up a bleach stain will not work, because the light spot remains lighter than the unbleached part even after dyeing. See "How can I fix the bleach spots on my favorite clothing?". Often the best answer is to find a close color match in a fabric marking pen, and use it to color in the spot.
(Please help support this web site. Thank you.)
Message: I got bleach on my new area rug, what can I use to put the light grey color back?
There is a product that I have never tried that claims to redye bleach stains on carpets to their original color. See http://www.ecarpetstains.com/. I cannot vouch for how well it may work, since I have not used it myself. The advantage of this treatment is that it claims to be usable on just the bleach stain itself.
As a general rule, trying to dye an entire item to cover up a bleach stain will not work, because the light spot remains lighter than the unbleached part even after dyeing. See "How can I fix the bleach spots on my favorite clothing?". Often the best answer is to find a close color match in a fabric marking pen, and use it to color in the spot.
(Please help support this web site. Thank you.)
Each grade is picking one color for their tie-dye shirt. I was wondering what process you thought would be the safest and easiest to use?
Name: Amanda
Message: Hello, I have been researching tie dye for some time and I have come across many different techniques. I am an elementary art teacher and I am wanting to tie dye shirts this year with my students (all grades k-6). We are using our tie dye shirts for field day (the last day of school outside activites). Each grade is picking one color for their tie-dye shirt. I was wondering what process you thought would be the safest and easiest to use? I am on a limited budget so I would also need the least expensive (for 900 kids). I thought of using the kool-aid tie dye for the younger students. But I would really like your thoughts on what you think would work best for the older and younger students. Thank you for your time!
The cheapest dye will be that purchased in bulk. You will want to use a fiber reactive dye such as Procion MX dye, purchased in 8-ounce or one-pound jars of each color. One ounce of this dye can be used to dye about two pounds of cotton fabric, so one pound of this dye can be used to tie-dye about thirty-two pounds of cotton shirts, or over a hundred children's size large t-shirts. Surprisingly, the best quality dye is far less expensive, when purchased by mail-order, than the poor quality all-purpose dye sold in many grocery stores and pharmacies in the US. It is also much easier to use, since you can use room-temperature water instead of having to use hot water (all-purpose dye works best in water that has been heated to just below boiling, and kept that hot, for half an hour, with the shirts in it).
Since each grade will be using just one color of dye, there is no point in having the students squirt on the dyes themselves in a tie-dye party. (I've seen enough rowdy misbehaving kids that I can't say I completely favor that idea, anyway.) Instead, you can have them each label their shirts (perhaps with a permanent black laundry marker, or by pinning on a tag cut from Tyvek mailing envelopes), then tie their shirts as they please, using rubber bands, string, waxed dental floss, or (my favorite) artificial sinew. There are many different possible patterns that they could make; you will want to make hand-outs for the students describing them. See, for example, the drawings on ProChem's "Learn Folding Techniques for Tie Dye". My children's elementary school chooses a different solid color t-shirt for each class, each year, unfortunately without tie-dyeing; using the identical color but different dyeing patterns will be much more interesting while still having great advantages (especially on field trips, when having the children each wear the same color makes it much easier to keep track of them).
You can then dump the shirts into the dye yourself, or have them watch while you do it. A very easy way to do the dyeing is by dropping the tied shirts into a washing machine with soda ash and salt, resetting the timer on the machine every twelve minutes or as needed to keep the dye and chemicals from draining for an hour or so. It is possible to use a large plastic trash can, but it will be no fun to dump such a large and heavy container full of dye water when you are done. If you do not use a washing machine, choose your container according to how much water you can easily move. A standard-sized top-loading washing machine can hold twenty gallons of water and dye 8 pounds of shirts at one time. However, since you are not trying to get a perfectly smooth even solid color, you do not need the large excess of water of a washing machine load, so any large bucket will work. Afterwards, you can remove the ties using blunt-ended children's scissors (to reduce the chance of accidentally cutting fabric), then rinse the shirts with cool water and finally wash out the excess dye with hot water and detergent, in the same washing machine, before going on to the next color.
Kool-aid tie-dyeing is a fun project, but it does not work on cotton shirts, so it is not practical for children's clothing. It's only for silk, wool, and some nylon. Unfortunately, food coloring cannot be used on cotton, as this kind of dye cannot bind to the cellulose fiber and washes out pretty quickly. Try it on cheap silk handkerchiefs, which can cost as little as $6 per dozen.
To buy the good Procion MX fiber reactive dye, you can mail-order from most of the companies listed on my Dye Sources Around the World page, or, for similar prices on 8 to 16 ounce jars of dye, you can order through Amazon using any of the following links, which will help to support my web site by providing a commission to my site at no additional cost to you:
Eight ounce jars of Procion MX dye through Amazon
One pound jars of Procion MX dye through Amazon
Color Chips for Choosing Procion MX dye Colors sold through Amazon (tiny jars only)
Color Chips for Choosing Different Sizes of Procion MX dye Colors sold through Amazon (under construction, to include links to all jar sizes)
You will also need soda ash, which you can buy with your dye or as sodium carbonate from the pool supply store. (Avoid sodium bicarbonate.)
(Please help support this web site. Thank you.)
Message: Hello, I have been researching tie dye for some time and I have come across many different techniques. I am an elementary art teacher and I am wanting to tie dye shirts this year with my students (all grades k-6). We are using our tie dye shirts for field day (the last day of school outside activites). Each grade is picking one color for their tie-dye shirt. I was wondering what process you thought would be the safest and easiest to use? I am on a limited budget so I would also need the least expensive (for 900 kids). I thought of using the kool-aid tie dye for the younger students. But I would really like your thoughts on what you think would work best for the older and younger students. Thank you for your time!
The cheapest dye will be that purchased in bulk. You will want to use a fiber reactive dye such as Procion MX dye, purchased in 8-ounce or one-pound jars of each color. One ounce of this dye can be used to dye about two pounds of cotton fabric, so one pound of this dye can be used to tie-dye about thirty-two pounds of cotton shirts, or over a hundred children's size large t-shirts. Surprisingly, the best quality dye is far less expensive, when purchased by mail-order, than the poor quality all-purpose dye sold in many grocery stores and pharmacies in the US. It is also much easier to use, since you can use room-temperature water instead of having to use hot water (all-purpose dye works best in water that has been heated to just below boiling, and kept that hot, for half an hour, with the shirts in it).
Since each grade will be using just one color of dye, there is no point in having the students squirt on the dyes themselves in a tie-dye party. (I've seen enough rowdy misbehaving kids that I can't say I completely favor that idea, anyway.) Instead, you can have them each label their shirts (perhaps with a permanent black laundry marker, or by pinning on a tag cut from Tyvek mailing envelopes), then tie their shirts as they please, using rubber bands, string, waxed dental floss, or (my favorite) artificial sinew. There are many different possible patterns that they could make; you will want to make hand-outs for the students describing them. See, for example, the drawings on ProChem's "Learn Folding Techniques for Tie Dye". My children's elementary school chooses a different solid color t-shirt for each class, each year, unfortunately without tie-dyeing; using the identical color but different dyeing patterns will be much more interesting while still having great advantages (especially on field trips, when having the children each wear the same color makes it much easier to keep track of them).
You can then dump the shirts into the dye yourself, or have them watch while you do it. A very easy way to do the dyeing is by dropping the tied shirts into a washing machine with soda ash and salt, resetting the timer on the machine every twelve minutes or as needed to keep the dye and chemicals from draining for an hour or so. It is possible to use a large plastic trash can, but it will be no fun to dump such a large and heavy container full of dye water when you are done. If you do not use a washing machine, choose your container according to how much water you can easily move. A standard-sized top-loading washing machine can hold twenty gallons of water and dye 8 pounds of shirts at one time. However, since you are not trying to get a perfectly smooth even solid color, you do not need the large excess of water of a washing machine load, so any large bucket will work. Afterwards, you can remove the ties using blunt-ended children's scissors (to reduce the chance of accidentally cutting fabric), then rinse the shirts with cool water and finally wash out the excess dye with hot water and detergent, in the same washing machine, before going on to the next color.
Kool-aid tie-dyeing is a fun project, but it does not work on cotton shirts, so it is not practical for children's clothing. It's only for silk, wool, and some nylon. Unfortunately, food coloring cannot be used on cotton, as this kind of dye cannot bind to the cellulose fiber and washes out pretty quickly. Try it on cheap silk handkerchiefs, which can cost as little as $6 per dozen.
To buy the good Procion MX fiber reactive dye, you can mail-order from most of the companies listed on my Dye Sources Around the World page, or, for similar prices on 8 to 16 ounce jars of dye, you can order through Amazon using any of the following links, which will help to support my web site by providing a commission to my site at no additional cost to you:
Eight ounce jars of Procion MX dye through Amazon
One pound jars of Procion MX dye through Amazon
Color Chips for Choosing Procion MX dye Colors sold through Amazon (tiny jars only)
Color Chips for Choosing Different Sizes of Procion MX dye Colors sold through Amazon (under construction, to include links to all jar sizes)
You will also need soda ash, which you can buy with your dye or as sodium carbonate from the pool supply store. (Avoid sodium bicarbonate.)
(Please help support this web site. Thank you.)
Monday, July 30, 2007
can I correct bleach damage on a spandex blend blouse?
Name: Christa
Message: HELP! I recently purchased an adorable white blouse(95% cotton and 5% spandex) with an army green woven combo (97% cotton and 3% spandex). When I bought it there was makeup on the white blouse so I spot treated it with a bleach pen. I washed it in the washing machine but on handwash cyle. When I took it out, the shoulder and back of neck have a yellowed bleach spot. How can I correct this?
I'm afraid that it is impossible to correct the damage in this case. Synthetic fibers such as spandex are permanently damaged by exposure to the hypochlorite in chlorine bleach. It is important to never use chlorine bleach on synthetic fibers.
A better choice to remove makeup stains from a spandex blend would be a bleach-free stain remover, makeup remover, or even white toothpaste. Color-safe non-chlorine "oxygen" bleaches, based on peroxide or perborate, are also suitable for use on spandex.
(Please help support this web site. Thank you.)
Message: HELP! I recently purchased an adorable white blouse(95% cotton and 5% spandex) with an army green woven combo (97% cotton and 3% spandex). When I bought it there was makeup on the white blouse so I spot treated it with a bleach pen. I washed it in the washing machine but on handwash cyle. When I took it out, the shoulder and back of neck have a yellowed bleach spot. How can I correct this?
I'm afraid that it is impossible to correct the damage in this case. Synthetic fibers such as spandex are permanently damaged by exposure to the hypochlorite in chlorine bleach. It is important to never use chlorine bleach on synthetic fibers.
A better choice to remove makeup stains from a spandex blend would be a bleach-free stain remover, makeup remover, or even white toothpaste. Color-safe non-chlorine "oxygen" bleaches, based on peroxide or perborate, are also suitable for use on spandex.
(Please help support this web site. Thank you.)
Sunday, July 29, 2007
why do yellow candles burn faster than white, blue, or red candles?
Hello Paula, I hope you can help as you are very busy answering so
many
questions.
—ADVERTISEMENTS—
My 14yo son is doing a science project (but of course I’m the
one making the enquiries). He has melted some candles and has
found the yellow ones burn quickest. (Wow). He used white, yellow, blue
and red.
The candle company (Australia) where we bought them said they use wax
soluble batik dyes. My limited understanding is that these are powder dyes
that are mixed with water and therefore become ‘solvent’?
Perhaps the batik dye is not made solvent with water, they just use it
straight?
However, apparently solvent dyes burn quicker.
My main question is what is Batik Dye? is it a Procion MX fibre
reactive dye? (which I thought was water soluble) and would it be used in making
candles?
I have searched your extensive website and blogs for these answers
but have become confused.
We actually need the chemical name for them (eg: Yellow MX 4G if this
is the Batik Dye name) and then we can research this and perhaps find a reason
why it would make the candle burn faster.
I don’t think the candle company wants to give out too much
info so any help you could give would be very much appreciated as you seem to
know everything about dyes.
This is a difficult question, because there are hundreds of different solvent dyes, and there is no way to narrow down which ones you have there.
The first thing to know is that substances that are soluble in wax are not soluble in water. Think about the oil and vinegar in a salad dressing, how they never mix together for long, no matter how you shake them. Vinegar is mostly water. If you add a drop of liquid food coloring to a mixture of oil and water, and shake it, the water (or vinegar) will be colored, but the oil will be unchanged. If you get an oil-soluble dye, instead, such as annatto seeds, their color will go into the oil part of the mixture, leaving the water or vinegar unchanged. As a general rule, a dye can either be soluble in oils, waxes, and fats, OR it can be soluble in water. You cannot use the same dye in water that you use in wax.
The word used to describe things that can be dissolve in water is hydrophilic, which means "water loving". The word used for things that can be dissolved in oil or wax is hydrophobic, which means "water hating". Solvent dyes can be described as hydrophobic dyes.
"Wax soluble batik dyes" is not a meaningful phrase to me. The dye is either going to be soluble in wax, and thus useful for candles, or it is going to be soluble in water, and thus usable for genuine batik. Maybe, just possibly, there might be some process somewhere that uses wax to apply oil-soluble dyes to fabric, but if so, I have never heard of it and cannot tell you about it. None of the dyes described on my web site, such as Procion MX dyes or acid dyes, are suitable for use in coloring wax for candles, as they are all hydrophilic substances which will dissolve in water, but not in wax. Procion MX and similar dyes that we use for batik are not soluble in wax.
The way the wax in batik works is by repelling dye that is dissolved in water. We use colorless wax for this purpose. We immerse the fabric in a water plus water-soluble dye dyebath, then remove it from the dyebath, rinse out and dry, then apply more wax to the fabric. Wherever wax has been applied, no more color goes. We never use wax-soluble dyes in batik, only water-soluble dyes.
Here is a link to a dye manufacturer's list of solvent dyes, just to show you how impossible it will be to get the correct name for the exact dye in the candles your son used. There are so many different solvent dyes that we cannot even guess which ones may have been used in your candles. Candles can also be dyed with insoluble pigments, but if there is any meaning to what the candle seller told you, your candles are colored with dyes, not pigments.
Different candles may burn at different speeds for a reason other than the specific dye. A candle that is a lighter color might have less dye added to the candle recipe and therefore contain a higher proportion of wax in the recipe.
Here is a more official explanation, quoted from the April 26, 2007 online issue of the newspaper "USA Today" :
This is a difficult question, because there are hundreds of different solvent dyes, and there is no way to narrow down which ones you have there.
The first thing to know is that substances that are soluble in wax are not soluble in water. Think about the oil and vinegar in a salad dressing, how they never mix together for long, no matter how you shake them. Vinegar is mostly water. If you add a drop of liquid food coloring to a mixture of oil and water, and shake it, the water (or vinegar) will be colored, but the oil will be unchanged. If you get an oil-soluble dye, instead, such as annatto seeds, their color will go into the oil part of the mixture, leaving the water or vinegar unchanged. As a general rule, a dye can either be soluble in oils, waxes, and fats, OR it can be soluble in water. You cannot use the same dye in water that you use in wax.
The word used to describe things that can be dissolve in water is hydrophilic, which means "water loving". The word used for things that can be dissolved in oil or wax is hydrophobic, which means "water hating". Solvent dyes can be described as hydrophobic dyes.
"Wax soluble batik dyes" is not a meaningful phrase to me. The dye is either going to be soluble in wax, and thus useful for candles, or it is going to be soluble in water, and thus usable for genuine batik. Maybe, just possibly, there might be some process somewhere that uses wax to apply oil-soluble dyes to fabric, but if so, I have never heard of it and cannot tell you about it. None of the dyes described on my web site, such as Procion MX dyes or acid dyes, are suitable for use in coloring wax for candles, as they are all hydrophilic substances which will dissolve in water, but not in wax. Procion MX and similar dyes that we use for batik are not soluble in wax.
The way the wax in batik works is by repelling dye that is dissolved in water. We use colorless wax for this purpose. We immerse the fabric in a water plus water-soluble dye dyebath, then remove it from the dyebath, rinse out and dry, then apply more wax to the fabric. Wherever wax has been applied, no more color goes. We never use wax-soluble dyes in batik, only water-soluble dyes.
Here is a link to a dye manufacturer's list of solvent dyes, just to show you how impossible it will be to get the correct name for the exact dye in the candles your son used. There are so many different solvent dyes that we cannot even guess which ones may have been used in your candles. Candles can also be dyed with insoluble pigments, but if there is any meaning to what the candle seller told you, your candles are colored with dyes, not pigments.
Different candles may burn at different speeds for a reason other than the specific dye. A candle that is a lighter color might have less dye added to the candle recipe and therefore contain a higher proportion of wax in the recipe.
Here is a more official explanation, quoted from the April 26, 2007 online issue of the newspaper "USA Today" :
Color makes no difference how fast a candle burns. "Black candles
burn no faster than white," says chandler Stefan
Phillips of the Island Candle Company.
Wick size is the primary factor determining candle-burning rate. "A
larger wick is like stepping on the gas pedal," says Phillips. The big wick
delivers more fuel to the burning flame.
By the way, a candle is a cylinder of solid fuel — paraffin wax
— that surrounds a wick. How does it burn? Bringing a lit match to a wick
melts and then vaporizes the wax coating the wick. The wax vapor combines with
oxygen, and burns.
Wax is the most important ingredient that makes a candle burn faster.
Soft wax has a higher oil content and lower melt temperature; therefore, it
burns faster. Typically, candles in jars have soft wax, and pillar candles have
hard wax. The longest burning candles are pillar candles made of a blend of
beeswax and paraffin.
Why would one color of candle have a different thickness of wick than another? Probably just chance. All of the candles of one color will be made in one batch, while the candles of another color will be prepared separately, in another batch. Perhaps the wicks just happened to be a little thicker, or thinner, the day the yellow candles were made.
Here is a link to a completely different explanation, claiming that darker colors might burn faster because the color black absorbs more light energy. I am not convinced that the result of color color absorption would be great enough to be measurable, however. I favor the wick hypothesis as the best guess at an explanation for why some candles burn faster than others.
GenWax.com has a thoughtfully answered FAQ section which says that the wick's capacity will overcome any effects of colors or scents. You will want to look at their answers.
(Please help support this web site. Thank you.)
Why would one color of candle have a different thickness of wick than another? Probably just chance. All of the candles of one color will be made in one batch, while the candles of another color will be prepared separately, in another batch. Perhaps the wicks just happened to be a little thicker, or thinner, the day the yellow candles were made.
Here is a link to a completely different explanation, claiming that darker colors might burn faster because the color black absorbs more light energy. I am not convinced that the result of color color absorption would be great enough to be measurable, however. I favor the wick hypothesis as the best guess at an explanation for why some candles burn faster than others.
GenWax.com has a thoughtfully answered FAQ section which says that the wick's capacity will overcome any effects of colors or scents. You will want to look at their answers.
(Please help support this web site. Thank you.)
Friday, July 27, 2007
I have a printed dress that I would like to dye a solid color. Must I remove the color first? and how??
Name: Elane
Message: I have a printed dress that I would like to dye a solid color. Must I remove the color first? and how??
It may or may not be possible to remove the printed design. It may have been printed with dyes, or it may have been printed with pigments. Many dyes cannot be bleached, but if your dress is 100% cotton or linen, you can try bleaching it to remove the design. There is no way to predict whether or not it will work. Do not use bleach on synthetic fibers, nor on protein fibers such as silk or wool. Another dye removal chemical, Rit Color Remover, can be used on fibers other than cotton; it, too, will work on some dyes but not others. See "What chemicals can be used to remove dye?".
If you do not remove the printed design before you dye, it will always be visible under the dye that you use to change the color of your dress. This is not necessarily a bad thing. Depending on the color you choose, the results can be quite attractive. Keep in mind that all dyes are transparent and will just add on to the existing color; they will not completely cover it up.
(Please help support this web site. Thank you.)
Message: I have a printed dress that I would like to dye a solid color. Must I remove the color first? and how??
It may or may not be possible to remove the printed design. It may have been printed with dyes, or it may have been printed with pigments. Many dyes cannot be bleached, but if your dress is 100% cotton or linen, you can try bleaching it to remove the design. There is no way to predict whether or not it will work. Do not use bleach on synthetic fibers, nor on protein fibers such as silk or wool. Another dye removal chemical, Rit Color Remover, can be used on fibers other than cotton; it, too, will work on some dyes but not others. See "What chemicals can be used to remove dye?".
If you do not remove the printed design before you dye, it will always be visible under the dye that you use to change the color of your dress. This is not necessarily a bad thing. Depending on the color you choose, the results can be quite attractive. Keep in mind that all dyes are transparent and will just add on to the existing color; they will not completely cover it up.
(Please help support this web site. Thank you.)
Thursday, July 26, 2007
I made the mistake of buying my bridesmaid dresses on-line. They are a red burgundy and I was shooting for a deeper color, more of a wine or egg plant color. The fabric is polyester satin. What can I use to dye this?
Name: Julie
Message: Hi there,
I made the mistake of buying my bridesmaid dresses on-line. They are a red burgundy and I was shooting for a deeper color, more of a wine or egg plant color. The fabric is polyester satin. What can I use to dye this and in your opinion is it possible?
No. You cannot dye anything that is not washable, and dyeing polyester requires extended boiling, which would probably destroy the trim on your dresses.
See the following link: "Dyeing Polyester with Disperse Dyes".
Polyester cannot be dyed with ordinary dyes, only with special polyester dyes. You must also use a toxic carrier chemical to make up for the fact that even boiling temperaures are not high enough in themselves. To use polyester dyes, you must have an enormous non-aluminum cooking pot, large enough for the garment to move freely as it boils, as otherwise the coloring will be uneven. This cooking pot should never again be used for food after you have used it for dyeing.
(Please help support this web site. Thank you.)
Message: Hi there,
I made the mistake of buying my bridesmaid dresses on-line. They are a red burgundy and I was shooting for a deeper color, more of a wine or egg plant color. The fabric is polyester satin. What can I use to dye this and in your opinion is it possible?
No. You cannot dye anything that is not washable, and dyeing polyester requires extended boiling, which would probably destroy the trim on your dresses.
See the following link: "Dyeing Polyester with Disperse Dyes".
Polyester cannot be dyed with ordinary dyes, only with special polyester dyes. You must also use a toxic carrier chemical to make up for the fact that even boiling temperaures are not high enough in themselves. To use polyester dyes, you must have an enormous non-aluminum cooking pot, large enough for the garment to move freely as it boils, as otherwise the coloring will be uneven. This cooking pot should never again be used for food after you have used it for dyeing.
(Please help support this web site. Thank you.)
Wednesday, July 25, 2007
I need to dye 100% olefin that has scotchgard on it.
Name: Debbie
Message: Hi, I need to dye 100% olefin that has a scotchgard on it. I work at an antique automobile restoration shop, and our upholstery department would like to know if they can remove the scotchgard off of the fabric with steam, and then attempt to dye the fabric. It is a natural color that we need to darken just a little to make more of a camel color. Thank you. If you could help at all I would appreciate it. What kind of dye do we need to use after removing the scotchgard?
I'm sorry, but olefin is not a dyeable fiber. Olefin is a name for polypropylene, which must be dyed before the liquid plastic is extruded into textile form. This is called "solution" dyeing, because the plastic is dyed while it is still dissolved in liquid. This method allows for much greater colorfastness, but it means that you cannot dye polypropylene yourself.
I am not sure that Scotchgard can be removed, even by steaming. However, in your case it does not matter, because even untreated olefin fabric cannot be dyed.
Polypropylene is an unusual fiber in that it is very hydrophobic, that is, it does not absorb water at all. I suspect that even fabric paint will not stick to this fiber properly.
(Please help support this web site. Thank you.)
Message: Hi, I need to dye 100% olefin that has a scotchgard on it. I work at an antique automobile restoration shop, and our upholstery department would like to know if they can remove the scotchgard off of the fabric with steam, and then attempt to dye the fabric. It is a natural color that we need to darken just a little to make more of a camel color. Thank you. If you could help at all I would appreciate it. What kind of dye do we need to use after removing the scotchgard?
I'm sorry, but olefin is not a dyeable fiber. Olefin is a name for polypropylene, which must be dyed before the liquid plastic is extruded into textile form. This is called "solution" dyeing, because the plastic is dyed while it is still dissolved in liquid. This method allows for much greater colorfastness, but it means that you cannot dye polypropylene yourself.
I am not sure that Scotchgard can be removed, even by steaming. However, in your case it does not matter, because even untreated olefin fabric cannot be dyed.
Polypropylene is an unusual fiber in that it is very hydrophobic, that is, it does not absorb water at all. I suspect that even fabric paint will not stick to this fiber properly.
(Please help support this web site. Thank you.)
Tuesday, July 24, 2007
I am trying to find Saxon Blue indigo liquid extract, as Trudy Van Stralen describes in her book Indigo Madder and Marigold. Do you use or know of a source for this liquid?
Name: Sheila
Message: Wonderful site! I am trying to find Saxon Blue indigo liquid extract, as Trudy Van Stralen describes in her book Indigo Madder & Marigold. Do you use or know of a source for this liquid?
What an interesting question! I do not have Van Stralen's book, but I do have Jim Liles' excellent Art and Craft of Natural Dyeing, which explains that Saxon Blue is indigo that has been dissolved by reacting it with concentrated sulfuric acid, creating an acid dye from what began as a vat dye.
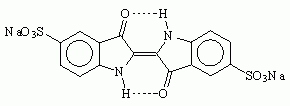
Another name for Saxon blue is Indigo Carmine. Now I have already heard about Indigo Carmine. It is a food coloring, US FD&C Blue No. 2, and is labeled E132 in Europe. Unfortunately, though it is very easy to find large quantities of FD&C blue 1 for sale as a food coloring, FD&C blue 2 seems to be much harder to come by. Interestingly, it is used medicinally in a test of kidney function. If you find a medical source, it will be vastly more expensive than a food coloring source. Another use of indigo carmine is as a pH indicator; it is blue at a pH of 11.6, but yellow at a pH of 13.0. One possible source of indigo carmine, sold for use as a biological stain, is Sargent Welch Chemistry, which sells a tiny 5-gram quantity for $8.50. Try Ward's Natural Science.
Saxon blue was discovered around 1740, according to Liles. He says that it dyes wool and silk without any need for a mordant, producing a striking greenish blue color, quit different from that of vatted indigo. It is not very lightfast, and fabrics dyed with it should not be subjected to strong sunlight. Using alum as a mordant might increase lightfastness and washfastness; Liles is not sure on this point.
Liles' recipe for making your own Saxon blue from indigo is as follows:
1. Into one pound of concentrated sulfuric acid (9 fluid
ounces), stir in slowly and by degrees 3 ounces of best
quality natural indigo or 1.5 ounces (8.5 level
tablespoons) of synthetic indigo. Make sure that the indigo
is very well ground before adding it to the acid (this
applies, primarily, to natural indigo). This should be done
in a strong heat-resistant glass vessel. Use a glass rod
for stirring.
2. Stir the mixture several times during the next few hours
and keep the mixture at about 100°F, if possible.
3. The next day, add gradually, stirring in slowly, about 1
teaspoon of chalk (optional).
4. Stir again the next day. At this point the preparation
is ready to use. Bottle the extract tightly and it will
keep at least a year or two.
5. [Liles wrote] I prefer to keep the product in a rather wide-mouthed
bottle with a good, tight sealing lid so that the material
may be removed by the spoonful.
Note that concentrated sulfuric acid is a highly caustic and dangerous chemical, best used only by those with training in working safely with chemicals. If I were to follow this recipe, I would wear a heavy coated apron, heavy thick rubber gloves, and a full plastic face shield, in case the sulfuric acid splatters. I would not be concerned about carcinogenicity or long-term toxic effects, though, only about the strong acid. None of the ingredients in the above recipeare poisonous once they have been diluted with large enough amounts of water to increase the pH to something reasonably close to neutral, but the concentrated sulfuric acid is such as strong acid as to warrant care in handling.
Liles also gives recipes for using Saxon blue to dye silk or wool, and another recipe for using it to dye cotton or linen. The latter, he says, is not very satisfactory and was used only for making greens, by overdyeing with yellow. Apparently the use of Saxon blue on silk or wool is quite satisfactory, however, as long as you do not expose the resulting fabric to bright light or sunlight very much.
(Please help support this web site. Thank you.)
Message: Wonderful site! I am trying to find Saxon Blue indigo liquid extract, as Trudy Van Stralen describes in her book Indigo Madder & Marigold. Do you use or know of a source for this liquid?
What an interesting question! I do not have Van Stralen's book, but I do have Jim Liles' excellent Art and Craft of Natural Dyeing, which explains that Saxon Blue is indigo that has been dissolved by reacting it with concentrated sulfuric acid, creating an acid dye from what began as a vat dye.

Another name for Saxon blue is Indigo Carmine. Now I have already heard about Indigo Carmine. It is a food coloring, US FD&C Blue No. 2, and is labeled E132 in Europe. Unfortunately, though it is very easy to find large quantities of FD&C blue 1 for sale as a food coloring, FD&C blue 2 seems to be much harder to come by. Interestingly, it is used medicinally in a test of kidney function. If you find a medical source, it will be vastly more expensive than a food coloring source. Another use of indigo carmine is as a pH indicator; it is blue at a pH of 11.6, but yellow at a pH of 13.0. One possible source of indigo carmine, sold for use as a biological stain, is Sargent Welch Chemistry, which sells a tiny 5-gram quantity for $8.50. Try Ward's Natural Science.
Saxon blue was discovered around 1740, according to Liles. He says that it dyes wool and silk without any need for a mordant, producing a striking greenish blue color, quit different from that of vatted indigo. It is not very lightfast, and fabrics dyed with it should not be subjected to strong sunlight. Using alum as a mordant might increase lightfastness and washfastness; Liles is not sure on this point.
Liles' recipe for making your own Saxon blue from indigo is as follows:
1. Into one pound of concentrated sulfuric acid (9 fluid
ounces), stir in slowly and by degrees 3 ounces of best
quality natural indigo or 1.5 ounces (8.5 level
tablespoons) of synthetic indigo. Make sure that the indigo
is very well ground before adding it to the acid (this
applies, primarily, to natural indigo). This should be done
in a strong heat-resistant glass vessel. Use a glass rod
for stirring.
2. Stir the mixture several times during the next few hours
and keep the mixture at about 100°F, if possible.
3. The next day, add gradually, stirring in slowly, about 1
teaspoon of chalk (optional).
4. Stir again the next day. At this point the preparation
is ready to use. Bottle the extract tightly and it will
keep at least a year or two.
5. [Liles wrote] I prefer to keep the product in a rather wide-mouthed
bottle with a good, tight sealing lid so that the material
may be removed by the spoonful.
Note that concentrated sulfuric acid is a highly caustic and dangerous chemical, best used only by those with training in working safely with chemicals. If I were to follow this recipe, I would wear a heavy coated apron, heavy thick rubber gloves, and a full plastic face shield, in case the sulfuric acid splatters. I would not be concerned about carcinogenicity or long-term toxic effects, though, only about the strong acid. None of the ingredients in the above recipeare poisonous once they have been diluted with large enough amounts of water to increase the pH to something reasonably close to neutral, but the concentrated sulfuric acid is such as strong acid as to warrant care in handling.
Liles also gives recipes for using Saxon blue to dye silk or wool, and another recipe for using it to dye cotton or linen. The latter, he says, is not very satisfactory and was used only for making greens, by overdyeing with yellow. Apparently the use of Saxon blue on silk or wool is quite satisfactory, however, as long as you do not expose the resulting fabric to bright light or sunlight very much.
(Please help support this web site. Thank you.)
Monday, July 23, 2007
Is there any way to create a custom camouflage pattern with natural dyes (they give a better tone for my purposes), such that the pattern will neither wash out nor leak in the rain?
Name: Mary Lynne
Message: Is there any way to create a custom camouflage pattern with natural dyes (they give a better tone for my purposes), such that the pattern will neither wash out nor leak in the rain? All of the natural dyes I am familiar with need to be soaked in hot water, and when i tried painting them on, they simply washed out. I have also tried commercial dyes, which were supposed to be for cold-water dyeing, but the colourfastness wore off after several washes. Are there perhaps any natural dyes suitable for cooler dyebaths, that might work with wax designs? I am particularly looking for greens, greys, and browns.
Colorfast or natural? Pick one. Natural dyes in general will not survive a great many machine washings. Most natural dyes cannot simply be painted on, or soaked in hot water; you must mordant your fabric first by boiling it in the mordant, and then apply the dye by boiling the fabric extensively in the dye. This process takes more than one day. If you are dyeing cotton, a good recipe calls for boiling the cotton in alum on day one, in tannic acid on day two, in alum again on day three, and finally in the natural dye on day four. (See The Dyer's Companion, by Dagmar Klos.) You can't expect permanent results by simply painting natural dyes on, as in almost all cases extensive simmering of the dyestuff with the fabric is necessary, using two or three times as much dyestuff, by weight, as you do fabric. Wool is easier to dye with natural dyes than cotton is, but I gather from your message that you are probably interested in dyeing cotton. The only natural dye I can recommend that will work with cool water, such as is required for wax batik, is indigo, an excellent traditional natural dye which is beyond the abilities of most beginners to use.
Some commercial dyes are noted for being poorly washfast. For example, all-purpose dye (sold under such brand names as Rit, Tintex, and Deka) must be applied by simmering the fabric in the dye for half an hour, for best results, and yet even then the dye will be poorly washfast unless it is treated with a cationic dye fixative such as Retayne.
In contrast, reactive dyes are extremely washfast and should retain their color through dozens of washings, if they are used correctly. Dylon Cold Water Dye and Dylon Permanent Dye are will last through hundreds of washings if they are applied according to the correct recipe, to the right kind of fabric. If you used either of these brands but had them wear off after only several washes, then there was something wrong with the way that you used them. If we discuss what you did, we can probably figure out what you did wrong. The best dyes to use yourself at home would be Procion MX dyes. It is extremely easy to make permanent camouflage effects by using Procion MX dyes with a technique called low water immersion dyeing. See "How to Do Low Water Immersion Dyeing".
Some people claim that natural dyes give better colors than synthetic dyes do, but in fact this is just because they do not understand how to mix colors. Any natural dye color can easily be duplicated with synthetic dyes, by adding greater or lessor amounts of the dye color that is opposite on the color wheel (such as by adding red to green, or orange to blue).
Be sure to use 100% cotton or other natural fiber, and use fabric that is free of finishes that may repel dye, such as stain resistance, water resistance, or no-iron finishes. Use fiber reactive dye with soda ash as a fixative, as recommended in the instructions (except for Dylon Permanent, which has the soda ash included in the dye powder).
Dyeing other materials requires different dyes and recipes. Some fibers, such as polyester, are impractical for home dyeing, though I can offer you one way to make an excellent camouflage design on polyester; others, such as polypropylene, or any fabric treated to be stain-resistant or water-repellant, are impossible to dye.
(Please help support this web site. Thank you.)
Message: Is there any way to create a custom camouflage pattern with natural dyes (they give a better tone for my purposes), such that the pattern will neither wash out nor leak in the rain? All of the natural dyes I am familiar with need to be soaked in hot water, and when i tried painting them on, they simply washed out. I have also tried commercial dyes, which were supposed to be for cold-water dyeing, but the colourfastness wore off after several washes. Are there perhaps any natural dyes suitable for cooler dyebaths, that might work with wax designs? I am particularly looking for greens, greys, and browns.
Colorfast or natural? Pick one. Natural dyes in general will not survive a great many machine washings. Most natural dyes cannot simply be painted on, or soaked in hot water; you must mordant your fabric first by boiling it in the mordant, and then apply the dye by boiling the fabric extensively in the dye. This process takes more than one day. If you are dyeing cotton, a good recipe calls for boiling the cotton in alum on day one, in tannic acid on day two, in alum again on day three, and finally in the natural dye on day four. (See The Dyer's Companion, by Dagmar Klos.) You can't expect permanent results by simply painting natural dyes on, as in almost all cases extensive simmering of the dyestuff with the fabric is necessary, using two or three times as much dyestuff, by weight, as you do fabric. Wool is easier to dye with natural dyes than cotton is, but I gather from your message that you are probably interested in dyeing cotton. The only natural dye I can recommend that will work with cool water, such as is required for wax batik, is indigo, an excellent traditional natural dye which is beyond the abilities of most beginners to use.
Some commercial dyes are noted for being poorly washfast. For example, all-purpose dye (sold under such brand names as Rit, Tintex, and Deka) must be applied by simmering the fabric in the dye for half an hour, for best results, and yet even then the dye will be poorly washfast unless it is treated with a cationic dye fixative such as Retayne.
In contrast, reactive dyes are extremely washfast and should retain their color through dozens of washings, if they are used correctly. Dylon Cold Water Dye and Dylon Permanent Dye are will last through hundreds of washings if they are applied according to the correct recipe, to the right kind of fabric. If you used either of these brands but had them wear off after only several washes, then there was something wrong with the way that you used them. If we discuss what you did, we can probably figure out what you did wrong. The best dyes to use yourself at home would be Procion MX dyes. It is extremely easy to make permanent camouflage effects by using Procion MX dyes with a technique called low water immersion dyeing. See "How to Do Low Water Immersion Dyeing".
Some people claim that natural dyes give better colors than synthetic dyes do, but in fact this is just because they do not understand how to mix colors. Any natural dye color can easily be duplicated with synthetic dyes, by adding greater or lessor amounts of the dye color that is opposite on the color wheel (such as by adding red to green, or orange to blue).
Be sure to use 100% cotton or other natural fiber, and use fabric that is free of finishes that may repel dye, such as stain resistance, water resistance, or no-iron finishes. Use fiber reactive dye with soda ash as a fixative, as recommended in the instructions (except for Dylon Permanent, which has the soda ash included in the dye powder).
Dyeing other materials requires different dyes and recipes. Some fibers, such as polyester, are impractical for home dyeing, though I can offer you one way to make an excellent camouflage design on polyester; others, such as polypropylene, or any fabric treated to be stain-resistant or water-repellant, are impossible to dye.
(Please help support this web site. Thank you.)
Sunday, July 22, 2007
My daughter and I are planning to dye 40 5.6 oz white cotton t-shirts. We are tie-dying them one color using RIT. How many boxes should we purchase?
Name: Carol
Message: Hi! My daughter and I are planning to dye 40 5.6 oz white cotton t-shirts. We are tie-dying them one color using RIT. How many boxes should we purchase? I searched the web (and a box of RIT) for answers but could not find anything other than 1 box is enough for 1 lb dry weight or 3 yards medium weight fabric. I thank you very much for your assistance.
Oh, dear. Please do not use all-purpose dye! You can buy much more satisfactory dye for a lot less money.
The way to figure out how much dye you need to use is by weighing your shirts. Get on a bathroom scale holding all forty of the shirts, and then without them, and subtract your weight from the total. Your total shirt weight might be twenty pounds, in which case you would need to use twenty boxes of Rit. At $2.79 per box, this will cost you $55! (For a dark shade, you should use two to four times as much dye, so you will need to increase the cost estimate if you want a dark shade. The Rit dye usage estimate assumes that you want a pale to medium shade.) The bad part is that all-purpose dye is a pain to use because it requires you to simmer the shirts in the dye for best results (washing machine temperatures are too low to work well with this dye), and, even then, the dye will fade quickly, and bleed forever in the laundry, unless you use a special dye fixative called Retayne, which you would probably have to buy by mail-order.
Instead, I strongly advise that you buy an easier-to-use, longer lasting, brighter, less expensive dye called Procion MX dye. For a single color, the easiest method is to follow the instructions in one of the links found on my "How to Dye in the Washing Machine" page. You can dye up to eight pounds of shirts at a time in most top-loading washing machines. Be sure to add exactly the same amount of dye, salt, soda ash, and t-shirts to each load so that the final dye color will match. Divide your twenty pounds of shirts into three loads of the same size.
How dark is the color you wish to dye? Just as for Rit dye, you will need to use more dye for a dark shade, less for a pastel shade. To dye twenty pounds of cotton t-shirts to a medium shade, you will need about eight ounces of Procion MX dye. An eight-ounce jar of Procion MX dye costs about twelve to fifteen dollars, plus shipping, but it varies by dye color. (If you use this link to order your Procion MX dye through Amazon, my site will receive a commission at no additional cost to you, or you can order it from most of the retailers listed on my Sources for Dye Supplies Around the World page.) You will also need a couple of pounds of soda ash (here's the link to buy soda ash through Amazon), and several pounds of non-iodized salt. If you decide to soak the shirts in soda ash and the squirt the dye on, as is usual for multi-colored tie-dye, you will not need salt. (See "How to Tie Dye".)
If you use a fiber reactive dye, such as Procion MX dye, instead of all-purpose dye, your work will be easier, your results will look much better, and the dye will last many times longer without fading on other clothes in the laundry. I will never again use all-purpose dye dye on cotton, because the results are so bad. I get a lot of sad emails from people who have used all-purpose dye and then want me to tell them how to make it work better.
(Please help support this web site. Thank you.)
Message: Hi! My daughter and I are planning to dye 40 5.6 oz white cotton t-shirts. We are tie-dying them one color using RIT. How many boxes should we purchase? I searched the web (and a box of RIT) for answers but could not find anything other than 1 box is enough for 1 lb dry weight or 3 yards medium weight fabric. I thank you very much for your assistance.
Oh, dear. Please do not use all-purpose dye! You can buy much more satisfactory dye for a lot less money.
The way to figure out how much dye you need to use is by weighing your shirts. Get on a bathroom scale holding all forty of the shirts, and then without them, and subtract your weight from the total. Your total shirt weight might be twenty pounds, in which case you would need to use twenty boxes of Rit. At $2.79 per box, this will cost you $55! (For a dark shade, you should use two to four times as much dye, so you will need to increase the cost estimate if you want a dark shade. The Rit dye usage estimate assumes that you want a pale to medium shade.) The bad part is that all-purpose dye is a pain to use because it requires you to simmer the shirts in the dye for best results (washing machine temperatures are too low to work well with this dye), and, even then, the dye will fade quickly, and bleed forever in the laundry, unless you use a special dye fixative called Retayne, which you would probably have to buy by mail-order.
Instead, I strongly advise that you buy an easier-to-use, longer lasting, brighter, less expensive dye called Procion MX dye. For a single color, the easiest method is to follow the instructions in one of the links found on my "How to Dye in the Washing Machine" page. You can dye up to eight pounds of shirts at a time in most top-loading washing machines. Be sure to add exactly the same amount of dye, salt, soda ash, and t-shirts to each load so that the final dye color will match. Divide your twenty pounds of shirts into three loads of the same size.
How dark is the color you wish to dye? Just as for Rit dye, you will need to use more dye for a dark shade, less for a pastel shade. To dye twenty pounds of cotton t-shirts to a medium shade, you will need about eight ounces of Procion MX dye. An eight-ounce jar of Procion MX dye costs about twelve to fifteen dollars, plus shipping, but it varies by dye color. (If you use this link to order your Procion MX dye through Amazon, my site will receive a commission at no additional cost to you, or you can order it from most of the retailers listed on my Sources for Dye Supplies Around the World page.) You will also need a couple of pounds of soda ash (here's the link to buy soda ash through Amazon), and several pounds of non-iodized salt. If you decide to soak the shirts in soda ash and the squirt the dye on, as is usual for multi-colored tie-dye, you will not need salt. (See "How to Tie Dye".)
If you use a fiber reactive dye, such as Procion MX dye, instead of all-purpose dye, your work will be easier, your results will look much better, and the dye will last many times longer without fading on other clothes in the laundry. I will never again use all-purpose dye dye on cotton, because the results are so bad. I get a lot of sad emails from people who have used all-purpose dye and then want me to tell them how to make it work better.
(Please help support this web site. Thank you.)
Friday, July 20, 2007
Do I have to presoak it again in the soda ash when I re-dye?
I've been reviewing your site to educate myself in garment dyeing
so I can dye a cotton skirt. I decided to tub dye and I presoaked it in
soda ash, then put the dye and salt in a bucket and then added some additional
soda ash per the instructions on the little bottle of dye I bought from
mister art.
Well, the color is perfect, but it is blotchy. My questions to you are:
Do I have to presoak it again in the soda ash when I re-dye it?
Do I need to add salt?
Do I need to add the soda ash after 20 minutes as per the instructions?
I'm going to try this time in my washing machine, is there anything else I can do to have the color come out even?
Thank you for your time. Your website is fabulous and very helpful.
First, what is the brand name of the dye you bought from mister art?
What does the label say?
It's Procion MX dye, chocolate brown.
That should work well. It does take an awful lot of stirring to get a smooth color by dyeing a garment in a bucket. Lots of extra water, too. Washing machine dyeing makes it a lot easier to get a solid color, since the machine does the stirring for you.
It's difficult to cover up a severe case of blotchiness, because the dark parts will stay darker and the light parts lighter, though both will be darker than before. The problem is similar to the Bleach Spot Problem. Large amounts of dye in a dark color can do it, or you can use Rit Color Remover (you'll need several packets in the washing machine) to lighten the skirt before trying again.
I should point out that there are also other possible causes of blotchiness: permanent press or stain-resistant finishes, invisible flaws in the fabric, or inadequate prewashing. Inadequate stirring is the most likely, however.
Once a garment has been washed out, the soda ash is all gone, so you will have to add more when dyeing it again. For dyeing in a bucket or washing machine, salt is necessary also, because otherwise too much of the dye stays in the water instead of going onto the fabric. Basically, the procedure for overdyeing a garment is exactly like dyeing it for the first time.
Be sure to get enough dye for your washing machine. A 16- or 20-gallon washing machine load requires more dye than a 5-gallon bucket. Check the required quantities for salt, soda ash, and dye. See the different recipes linked to on "How can I dye clothing or fabric in the washing machine?".
(Please help support this web site. Thank you.)
mister art.
Well, the color is perfect, but it is blotchy. My questions to you are:
Do I have to presoak it again in the soda ash when I re-dye it?
Do I need to add salt?
Do I need to add the soda ash after 20 minutes as per the instructions?
I'm going to try this time in my washing machine, is there anything else I can do to have the color come out even?
Thank you for your time. Your website is fabulous and very helpful.
First, what is the brand name of the dye you bought from mister art?
What does the label say?
It's Procion MX dye, chocolate brown.
That should work well. It does take an awful lot of stirring to get a smooth color by dyeing a garment in a bucket. Lots of extra water, too. Washing machine dyeing makes it a lot easier to get a solid color, since the machine does the stirring for you.
It's difficult to cover up a severe case of blotchiness, because the dark parts will stay darker and the light parts lighter, though both will be darker than before. The problem is similar to the Bleach Spot Problem. Large amounts of dye in a dark color can do it, or you can use Rit Color Remover (you'll need several packets in the washing machine) to lighten the skirt before trying again.
I should point out that there are also other possible causes of blotchiness: permanent press or stain-resistant finishes, invisible flaws in the fabric, or inadequate prewashing. Inadequate stirring is the most likely, however.
Once a garment has been washed out, the soda ash is all gone, so you will have to add more when dyeing it again. For dyeing in a bucket or washing machine, salt is necessary also, because otherwise too much of the dye stays in the water instead of going onto the fabric. Basically, the procedure for overdyeing a garment is exactly like dyeing it for the first time.
Be sure to get enough dye for your washing machine. A 16- or 20-gallon washing machine load requires more dye than a 5-gallon bucket. Check the required quantities for salt, soda ash, and dye. See the different recipes linked to on "How can I dye clothing or fabric in the washing machine?".
(Please help support this web site. Thank you.)
Wednesday, July 18, 2007
dyeing a cotton slipcover in a front-loading washing machine
Name: Kelly
Message: Hello,
I am considering dying a sofa cover (along with the cushion covers) and have a few questions. The cover is 100% cotton, made my Mitchell Gold and sold through Restoration Hardware a few years back. The color is a light, neutralized gold-yellow. I would like to change it to a light brown. I have a front-loading washer. I've read your information about dying with Dylon dyes and have found this brand of dye on the Dick Blick website. My questions are:
1. What do you think of this idea? Is it possible to get good results on something this large using this method? I don't have much experience with dye (just a bit of shibori).
2. Would I need to fit all of the covers in the same load in the washer? How much variability would there be if I needed to divide things up because of space limitations of the washing machine?
3. With the Dylon dyes, would I need to use the technique you mention, of delaying the end of the washing cycle so that more dye saturates the fabric before the spin cycle comes on?
4. Would I weigh the cover and cushion covers to determine the amount of dye to use, based on weight?
5. Would this process require the Dylon Cold Dye Fix that is also available on the Dick Blick website?
Dylon Washing Machine dye is a line of fiber reactive dye that is not available at all in the United States. Dick Blick does not sell Dylon Washing Machine dye. Dylon Cold Dye is a completely different kind of fiber reactive dye. Other fiber reactive dyes, including Dylon Cold Dye which is actually mostly Procion MX dye, can be used in a front-loading washing machine, if you are able to add the soda ash solution via a bleach or detergent dispenser. It depends on the settings that are available on your own particular model of front-loading machine. If everything must be added to the washer at once before starting the wash cycle, then there is a risk of uneven color, due to adding the soda ash too soon, before the dye has had a chance to penetrate the fabric. I have, however, seen instructions to put dye, salt, soda ash, and fabric into the drum of the washing machine at once, so it's not impossible to get good results that way.
It is possible to dye a 100% cotton slipcover satisfactorily with a cool water dye such as Procion MX dye, whether it's the Dylon brand or another one. People have done so and told me about it, though only in top-loading washing machines, not front-loaders. However, I am, like you, concerned about slight color variations between loads, if you have to split up the parts of the slipcover into two loads. You will probably be able to get a good enough match if you are meticulous in weighing out your dye and auxiliary chemicals (soda ash and salt), so that the exact same amount of dye per pound of fabric is used. It would be best if you can include the same weight of fabric in each load. How much does your slipcover weigh? You can dye up to eight pounds of cotton fabric in one top-loading washing machine load; consult the manual for your front-loading washing machine to find out its limits.
Using a cool water dye, such as Procion MX dye, is important if you need to be careful not to shrink the slipcovers. Slipcovers that have shrunk are practically useless. Do not use a hot water dye, such as Rit All-purpose dye, if your slipcover can be washed only in cool water.
Dylon Cold dye is mostly Procion MX dye. (Only a few of the colors contain one or two other dyes which are not Procion MX dyes.) It's good dye, but the tiny tins are expensive. Each 5-gram tin will dye only half a pound of material, and costs over $2. Compare this to the Procion MX dye sold by PRO Chemical & Dye (look under "MX Reactive Dyes"): a 56-gram jar costs from $2 to $6, depending on color, but contains over ten times as much dye. The MX dye from PRO Chemical & Dye is certainly not inferior to the Dylon Cold Dye; it might be superior due to greater freshness.
Dylon Cold Dye must be used with Dylon Cold Fix, or the same chemical, soda ash, under another name. One 15-gram packet of Dylon Cold Fix costs 89 cents, but one pound of soda ash from PRO Chemical, which is 454 grams, costs $1.75. Thirty times as much soda ash costs only twice as much. It is the same substance. Alternatively, yo can buy sodium carbonate at a pool supply store (do NOT get sodium bicarbonate instead). You will also need a pound or more of non-iodized salt. Look for ice cream salt or pickling salt at your grocery store. Any non-iodized salt will do; iodized salt is probably okay, but you probably don't want to take the chance.
Dharma Trading Company, another good source for two-ounce and eight-ounce jars of Procion MX dye, recommends adding Calsolene oil for washing machine dyeing. It might help to get the color a little more even on your fabric.
You must thoroughly prewash the covers so that no invisible stains remain to repel dye. It is amazing how a stain can be quite invisible before dyeing and very highly visible after dyeing. Prewash in the hottest water the covers can tolerate, with detergent and extra soda ash (to boost the cleaning power of the laundry detergent).
It is important that the slipcovers not have any finishes that will prevent dye from reaching the fabric, such as stain-resistance or permanent press finishes. Sometimes, rarely, you will find that different panels of fabric that match in color before dyeing end up taking the dye darker or lighter than each other, due to having come from different bolts of fabric. There is nothing to be done about this problem.
Regardless of dye type, you will get best results if you allow an hour for the dye to react with the cotton fabric, after you add the soda ash. This will require resetting the washing machine repeatedly, so the dye, salt, and soda ash do not drain away prematurely. I do not know whether or not your particular model of front-loading washer will allow this.
If you are in doubt about the amount of dye to use to get the light brown that you want, I recommend that you err on the lighter side, because it is much easier to dye again to get a darker color than it is to remove dye after you have used it. ProChem recommends that you use 13 grams of dye powder to dye five pounds of fabric a pale color.
Mixed dye colors, such as brown, should be dissolved in water and filtered through a coffee filter or piece of nylon stocking in a strainer before use, as tiny dye particles can make red dots on your fabric.
(Please help support this web site. Thank you.)
Message: Hello,
I am considering dying a sofa cover (along with the cushion covers) and have a few questions. The cover is 100% cotton, made my Mitchell Gold and sold through Restoration Hardware a few years back. The color is a light, neutralized gold-yellow. I would like to change it to a light brown. I have a front-loading washer. I've read your information about dying with Dylon dyes and have found this brand of dye on the Dick Blick website. My questions are:
1. What do you think of this idea? Is it possible to get good results on something this large using this method? I don't have much experience with dye (just a bit of shibori).
2. Would I need to fit all of the covers in the same load in the washer? How much variability would there be if I needed to divide things up because of space limitations of the washing machine?
3. With the Dylon dyes, would I need to use the technique you mention, of delaying the end of the washing cycle so that more dye saturates the fabric before the spin cycle comes on?
4. Would I weigh the cover and cushion covers to determine the amount of dye to use, based on weight?
5. Would this process require the Dylon Cold Dye Fix that is also available on the Dick Blick website?
Dylon Washing Machine dye is a line of fiber reactive dye that is not available at all in the United States. Dick Blick does not sell Dylon Washing Machine dye. Dylon Cold Dye is a completely different kind of fiber reactive dye. Other fiber reactive dyes, including Dylon Cold Dye which is actually mostly Procion MX dye, can be used in a front-loading washing machine, if you are able to add the soda ash solution via a bleach or detergent dispenser. It depends on the settings that are available on your own particular model of front-loading machine. If everything must be added to the washer at once before starting the wash cycle, then there is a risk of uneven color, due to adding the soda ash too soon, before the dye has had a chance to penetrate the fabric. I have, however, seen instructions to put dye, salt, soda ash, and fabric into the drum of the washing machine at once, so it's not impossible to get good results that way.
It is possible to dye a 100% cotton slipcover satisfactorily with a cool water dye such as Procion MX dye, whether it's the Dylon brand or another one. People have done so and told me about it, though only in top-loading washing machines, not front-loaders. However, I am, like you, concerned about slight color variations between loads, if you have to split up the parts of the slipcover into two loads. You will probably be able to get a good enough match if you are meticulous in weighing out your dye and auxiliary chemicals (soda ash and salt), so that the exact same amount of dye per pound of fabric is used. It would be best if you can include the same weight of fabric in each load. How much does your slipcover weigh? You can dye up to eight pounds of cotton fabric in one top-loading washing machine load; consult the manual for your front-loading washing machine to find out its limits.
Using a cool water dye, such as Procion MX dye, is important if you need to be careful not to shrink the slipcovers. Slipcovers that have shrunk are practically useless. Do not use a hot water dye, such as Rit All-purpose dye, if your slipcover can be washed only in cool water.
Dylon Cold dye is mostly Procion MX dye. (Only a few of the colors contain one or two other dyes which are not Procion MX dyes.) It's good dye, but the tiny tins are expensive. Each 5-gram tin will dye only half a pound of material, and costs over $2. Compare this to the Procion MX dye sold by PRO Chemical & Dye (look under "MX Reactive Dyes"): a 56-gram jar costs from $2 to $6, depending on color, but contains over ten times as much dye. The MX dye from PRO Chemical & Dye is certainly not inferior to the Dylon Cold Dye; it might be superior due to greater freshness.
Dylon Cold Dye must be used with Dylon Cold Fix, or the same chemical, soda ash, under another name. One 15-gram packet of Dylon Cold Fix costs 89 cents, but one pound of soda ash from PRO Chemical, which is 454 grams, costs $1.75. Thirty times as much soda ash costs only twice as much. It is the same substance. Alternatively, yo can buy sodium carbonate at a pool supply store (do NOT get sodium bicarbonate instead). You will also need a pound or more of non-iodized salt. Look for ice cream salt or pickling salt at your grocery store. Any non-iodized salt will do; iodized salt is probably okay, but you probably don't want to take the chance.
Dharma Trading Company, another good source for two-ounce and eight-ounce jars of Procion MX dye, recommends adding Calsolene oil for washing machine dyeing. It might help to get the color a little more even on your fabric.
You must thoroughly prewash the covers so that no invisible stains remain to repel dye. It is amazing how a stain can be quite invisible before dyeing and very highly visible after dyeing. Prewash in the hottest water the covers can tolerate, with detergent and extra soda ash (to boost the cleaning power of the laundry detergent).
It is important that the slipcovers not have any finishes that will prevent dye from reaching the fabric, such as stain-resistance or permanent press finishes. Sometimes, rarely, you will find that different panels of fabric that match in color before dyeing end up taking the dye darker or lighter than each other, due to having come from different bolts of fabric. There is nothing to be done about this problem.
Regardless of dye type, you will get best results if you allow an hour for the dye to react with the cotton fabric, after you add the soda ash. This will require resetting the washing machine repeatedly, so the dye, salt, and soda ash do not drain away prematurely. I do not know whether or not your particular model of front-loading washer will allow this.
If you are in doubt about the amount of dye to use to get the light brown that you want, I recommend that you err on the lighter side, because it is much easier to dye again to get a darker color than it is to remove dye after you have used it. ProChem recommends that you use 13 grams of dye powder to dye five pounds of fabric a pale color.
Mixed dye colors, such as brown, should be dissolved in water and filtered through a coffee filter or piece of nylon stocking in a strainer before use, as tiny dye particles can make red dots on your fabric.
(Please help support this web site. Thank you.)
Tuesday, July 17, 2007
What is cold water dye and how is it used? I have Dylon cold dye and I want to use it.
Name: Mehar
Message: What is cold water dye and how is it used? I have Dylon cold dye and I want to use it.
Cold water dye refers to any dye which does not require very hot water for fixation. It does not necessarily mean using water that feels cool to the touch.

Most of the dyes in the Dylon Cold Water Dyes line are Procion MX dyes; one or two are from other classes of cool water reactive dyes. They work well on cotton, linen, rayon, and silk; they will not work on polyester. See the Dye Forum posting from September 27, 2006, entitled "more about Dylon Cold Water Dyes".
Unlike Dylon Permanent Dyes, the Dylon Cold Dyes require the addition of separate dye fixative, which is either soda ash or another high-pH chemical. You can use the Dylon brand, called Dylon Cold Fix, or you can use any other brand of soda ash or sodium carbonate for this purpose.
The best temperature of water to use for dyeing with Dylon Cold Dye is between 75° to 95°F (24° to 35°C). Higher temperatures are also acceptable, but temperatures below 70°F (21°C) should be avoided.
The instructions on the "Hand Dyeing - How to Do It: basic recipe for Procion MX dyes on cellulose or silk" page will work fine with Dylon Cold Dye. (They must be amended slightly for use with Dylon Permanent dye, which is a different kind of reactive dye with the auxiliary chemicals already added.)
(Please help support this web site. Thank you.)
Message: What is cold water dye and how is it used? I have Dylon cold dye and I want to use it.
Cold water dye refers to any dye which does not require very hot water for fixation. It does not necessarily mean using water that feels cool to the touch.

Most of the dyes in the Dylon Cold Water Dyes line are Procion MX dyes; one or two are from other classes of cool water reactive dyes. They work well on cotton, linen, rayon, and silk; they will not work on polyester. See the Dye Forum posting from September 27, 2006, entitled "more about Dylon Cold Water Dyes".
Unlike Dylon Permanent Dyes, the Dylon Cold Dyes require the addition of separate dye fixative, which is either soda ash or another high-pH chemical. You can use the Dylon brand, called Dylon Cold Fix, or you can use any other brand of soda ash or sodium carbonate for this purpose.
The best temperature of water to use for dyeing with Dylon Cold Dye is between 75° to 95°F (24° to 35°C). Higher temperatures are also acceptable, but temperatures below 70°F (21°C) should be avoided.
The instructions on the "Hand Dyeing - How to Do It: basic recipe for Procion MX dyes on cellulose or silk" page will work fine with Dylon Cold Dye. (They must be amended slightly for use with Dylon Permanent dye, which is a different kind of reactive dye with the auxiliary chemicals already added.)
(Please help support this web site. Thank you.)
Monday, July 16, 2007
I have an evening dress that is 100% polyester BUT it says handwash in cold water. Can it be dyed? It's baby blue.
Name: Buttons
Message: ok, I got one. I have an evening dress that is 100% polyester BUT it says handwash in cold water. Can it be dyed? It's baby blue.
No, it can't. The only kind of dye that will work on polyester is Disperse Dye, which can be applied only at high temperatures. You would have to boil the dress for an hour with the dye. Since your dress can be washed in cool water only, not hot water, let alone boiling water, it cannot be expected to survive the dyeing process.
If you're planning to throw the dress away anyway, you might want to try dyeing it as an experiment; there's always a possibility that the dress might survive the boiling process. If so, you will need to find a large non-aluminum cooking pot, large enough that the dress can move freely when submerged in water. (The cooking pot should not be used for food again after it has been used as a dyepot.) You will need to order Disperse dyes from one of the limited number of dye companies that sells this special type of dye, such as PRO Chemical & Dye or Aljo Dyes in the US, Batik Oetoro in Australia, or Rainbow Silks in the UK. You cannot use all-purpose dye or another type of dye; only disperse dye will work. For medium to dark shades, you will also need the dye carrier chemical that your dye supplier sells; for light shades, you don't need to use the carrier chemical, which is smelly and toxic. See "Dyeing Polyester with Disperse Dyes".
Another possibility is to use fabric paint. You could use Dharma's Pigment Dye System (which, in spite of the name, is not dye at all) or Jacquard's Dye-na-flow. Fabric paint tends to feel a little stiffer than dye, but these two brands are among the softest and lightest of fabric paints, and they are both supposed to work pretty well on polyester, though they do not produce as smooth and even a color as a dye would. They are both ideal for tie-dyeing polyester. Unlike disperse dye, fabric paint needs only ironing to heat-set it, which is much easier on the fabric. You would not need an expensive cooking pot, and you would not be limited to a single color unless you want to be. What do you think? The Dharma Pigment Dye system can be purchased only by mail order (unless you live near San Rafael, California), but Dye-na-flow can be purchased in several good local crafts stores, in addition to being available by mail-order.
I would suggest diluting the paint as much as the instructions permit (for Dharma Pigment, dilute with two to four times as much water as you have paint; for Dye-na-flow, dilute with no more than one-quarter the volume of the paint you use). Put the diluted paint in an inexpensive bucket or other plastic container that you don't mind ruining, and immerse the dress in it and squeeze it around. Having more paint than you really need would make this a lot easier and help to make the color more even. In any case you will inevitably get some unevenness of coloring, but this can look pretty neat. Once the paint has penetrated the fabric, hang it up or lay it flat on some surface that you don't mind spoiling, such as an old shower curtain. Be careful of drips, which will ruin your floor or disfigure your sidewalk. (Wipe up any drips IMMEDIATELY, but plan ahead so they do not happen in the first place.) Let the dress dry throughly, which may take two or three days; when it is dry, heat-set as indicated in the instructions provided with the paint. Delay your first washing as long as possible, up to a month if convenient to do so, and then turn the dress inside out and wash by hand to help avoid rubbing off too much of the paint, which will tend to wear off.
You know what would be neat would be to use some good metallic fabric paint, such as Lumiere, or else add some Pearl Ex brand mica powder to the Dye-Na-Flow or Dharma Pigment dye. The binder in the pigment "dye" paint would hold the Pearl Ex sparkle dust stuff. Lumiere is a Jacquard fabric paint, and all Jacquard fabric paints are supposed to work on polyester. You'd get a somewhat mottled effect, which could be pretty cool.
(Please help support this web site. Thank you.)
Message: ok, I got one. I have an evening dress that is 100% polyester BUT it says handwash in cold water. Can it be dyed? It's baby blue.
No, it can't. The only kind of dye that will work on polyester is Disperse Dye, which can be applied only at high temperatures. You would have to boil the dress for an hour with the dye. Since your dress can be washed in cool water only, not hot water, let alone boiling water, it cannot be expected to survive the dyeing process.
If you're planning to throw the dress away anyway, you might want to try dyeing it as an experiment; there's always a possibility that the dress might survive the boiling process. If so, you will need to find a large non-aluminum cooking pot, large enough that the dress can move freely when submerged in water. (The cooking pot should not be used for food again after it has been used as a dyepot.) You will need to order Disperse dyes from one of the limited number of dye companies that sells this special type of dye, such as PRO Chemical & Dye or Aljo Dyes in the US, Batik Oetoro in Australia, or Rainbow Silks in the UK. You cannot use all-purpose dye or another type of dye; only disperse dye will work. For medium to dark shades, you will also need the dye carrier chemical that your dye supplier sells; for light shades, you don't need to use the carrier chemical, which is smelly and toxic. See "Dyeing Polyester with Disperse Dyes".
Another possibility is to use fabric paint. You could use Dharma's Pigment Dye System (which, in spite of the name, is not dye at all) or Jacquard's Dye-na-flow. Fabric paint tends to feel a little stiffer than dye, but these two brands are among the softest and lightest of fabric paints, and they are both supposed to work pretty well on polyester, though they do not produce as smooth and even a color as a dye would. They are both ideal for tie-dyeing polyester. Unlike disperse dye, fabric paint needs only ironing to heat-set it, which is much easier on the fabric. You would not need an expensive cooking pot, and you would not be limited to a single color unless you want to be. What do you think? The Dharma Pigment Dye system can be purchased only by mail order (unless you live near San Rafael, California), but Dye-na-flow can be purchased in several good local crafts stores, in addition to being available by mail-order.
I would suggest diluting the paint as much as the instructions permit (for Dharma Pigment, dilute with two to four times as much water as you have paint; for Dye-na-flow, dilute with no more than one-quarter the volume of the paint you use). Put the diluted paint in an inexpensive bucket or other plastic container that you don't mind ruining, and immerse the dress in it and squeeze it around. Having more paint than you really need would make this a lot easier and help to make the color more even. In any case you will inevitably get some unevenness of coloring, but this can look pretty neat. Once the paint has penetrated the fabric, hang it up or lay it flat on some surface that you don't mind spoiling, such as an old shower curtain. Be careful of drips, which will ruin your floor or disfigure your sidewalk. (Wipe up any drips IMMEDIATELY, but plan ahead so they do not happen in the first place.) Let the dress dry throughly, which may take two or three days; when it is dry, heat-set as indicated in the instructions provided with the paint. Delay your first washing as long as possible, up to a month if convenient to do so, and then turn the dress inside out and wash by hand to help avoid rubbing off too much of the paint, which will tend to wear off.
You know what would be neat would be to use some good metallic fabric paint, such as Lumiere, or else add some Pearl Ex brand mica powder to the Dye-Na-Flow or Dharma Pigment dye. The binder in the pigment "dye" paint would hold the Pearl Ex sparkle dust stuff. Lumiere is a Jacquard fabric paint, and all Jacquard fabric paints are supposed to work on polyester. You'd get a somewhat mottled effect, which could be pretty cool.
(Please help support this web site. Thank you.)
Sunday, July 15, 2007
Is it possible to dye red Cordura nylon fabric to yellow?
Name: Noah
Message: Hello, I found your site while searching about dying nylon. I am trying to find out if it is possible to dye red CORDORA fabric to Yellow and if so what dyes should I use? I am trying to find out because I am looking at a red Sparco kart racing suit that is red, I have been trying to find one in yellow but the one I am looking for is now out of production and sold out in the yellow color everywhere I look. I can get basically the same suit in red right now. Can this change in color be accomplished? http://www.kartpro.com/sparco/suits/imolacolors.jpg this picture shows a similar suit in the same shade or red, and it shows the shade of yellow I want. Can this be done or should I wait for a used suit in yellow? The only way I can get a suit in the yellow color as shown from a maker I trust (in racing you must use safety gear you trust) will cost about $250 more than I can get that red suit for. I would appreciate any help regarding my question.
This, unfortunately, is a pretty easy question to answer. No, you can't do it.
Dye is transparent, so every color you dye a garment with is just added on the the colors already in it. If you spend a little time with some watercolor paint, you will see that it is possible to cover up a yellow color with red, either by using red or by using magenta (which combines with the yellow to make red), but there is no way to use transparent color to turn something that is red into yellow.
Cordura is made of nylon and can be dyed with the use of acid dyes (see "About Acid Dyes"). This requires an acid dye, a cooking pot that is large enough for the garment to move freely but which will never be used again for food, and a mild acid such as vinegar. However, if there is any finish on the fabric, such as a water-repellant, stain-resistant, or permanent-press finish, the dye will not be able to reach the fabric evenly, so it cannot be dyed successfully.
It might be possible to discharge the color from a red Cordura suit, using a product called sodium dithionite, also known as sodium hydrosulfite, which is sold under the name Rit Color Remover. However, there is no predicting whether or not it will work. Some dyes cannot be discharged. In addition, I do not think that this treatment would reduce the strength of the nylon, but I can't guarantee it. Nylon absolutely should not ever be subjected to chlorine bleach, which is a more common discharge agent, but which is very damaging to synthetic fibers.
I think you had better pay the higher price for the suit that is already the color that you need. Sorry.
(Please help support this web site. Thank you.)
Message: Hello, I found your site while searching about dying nylon. I am trying to find out if it is possible to dye red CORDORA fabric to Yellow and if so what dyes should I use? I am trying to find out because I am looking at a red Sparco kart racing suit that is red, I have been trying to find one in yellow but the one I am looking for is now out of production and sold out in the yellow color everywhere I look. I can get basically the same suit in red right now. Can this change in color be accomplished? http://www.kartpro.com/sparco/suits/imolacolors.jpg this picture shows a similar suit in the same shade or red, and it shows the shade of yellow I want. Can this be done or should I wait for a used suit in yellow? The only way I can get a suit in the yellow color as shown from a maker I trust (in racing you must use safety gear you trust) will cost about $250 more than I can get that red suit for. I would appreciate any help regarding my question.
This, unfortunately, is a pretty easy question to answer. No, you can't do it.
Dye is transparent, so every color you dye a garment with is just added on the the colors already in it. If you spend a little time with some watercolor paint, you will see that it is possible to cover up a yellow color with red, either by using red or by using magenta (which combines with the yellow to make red), but there is no way to use transparent color to turn something that is red into yellow.
Cordura is made of nylon and can be dyed with the use of acid dyes (see "About Acid Dyes"). This requires an acid dye, a cooking pot that is large enough for the garment to move freely but which will never be used again for food, and a mild acid such as vinegar. However, if there is any finish on the fabric, such as a water-repellant, stain-resistant, or permanent-press finish, the dye will not be able to reach the fabric evenly, so it cannot be dyed successfully.
It might be possible to discharge the color from a red Cordura suit, using a product called sodium dithionite, also known as sodium hydrosulfite, which is sold under the name Rit Color Remover. However, there is no predicting whether or not it will work. Some dyes cannot be discharged. In addition, I do not think that this treatment would reduce the strength of the nylon, but I can't guarantee it. Nylon absolutely should not ever be subjected to chlorine bleach, which is a more common discharge agent, but which is very damaging to synthetic fibers.
I think you had better pay the higher price for the suit that is already the color that you need. Sorry.
(Please help support this web site. Thank you.)
Saturday, July 14, 2007
I have a lot of fifty to sixty year old linens that have turn yellow. Is there anyway that I can salvage them?
Name: barbara
Message: I have a lot of fifty to sixty year old linens that have turn yellow. Is there anyway that I can salvage them?
The most traditional way to whiten linens is to use the sun to bleach them. Just lay them on dry grass in the sunlight. (I've seen speculation that the grass plays some important chemical role, but, in fact, its only purpose in this case is to keep the sheets from getting in the dirt while they are lying out flat in the sunlight.)
Have you tried washing them? If ordinary laundry detergent does not do the job, try soaking them for a while, or use an oxygen bleach such as Oxy-Boost or Oxy-Clean. For valuable archival-quality linens, use only a very gentle pure detergent such as Orvus Paste.
To counteract the natural yellow of the fiber, you can use Mrs. Stewart's Bluing, which can usually be purchased from the grocery store, or, more effectively, modern fluorescent brighteners, sold as Rit brand Whitener and Brightener . Follow the package instructions. Both of these treatments are much gentler than bleaching. They are, in effect, temporary dyes. By reflecting blue light, they make the fabric appear to be whiter.
There is no cure for fabric that contains polyester and has been yellowed by bleach damage. Polyester cannot tolerate treatment with chlorine bleach.
(Please help support this web site. Thank you.)
Message: I have a lot of fifty to sixty year old linens that have turn yellow. Is there anyway that I can salvage them?
The most traditional way to whiten linens is to use the sun to bleach them. Just lay them on dry grass in the sunlight. (I've seen speculation that the grass plays some important chemical role, but, in fact, its only purpose in this case is to keep the sheets from getting in the dirt while they are lying out flat in the sunlight.)
Have you tried washing them? If ordinary laundry detergent does not do the job, try soaking them for a while, or use an oxygen bleach such as Oxy-Boost or Oxy-Clean. For valuable archival-quality linens, use only a very gentle pure detergent such as Orvus Paste.
To counteract the natural yellow of the fiber, you can use Mrs. Stewart's Bluing, which can usually be purchased from the grocery store, or, more effectively, modern fluorescent brighteners, sold as Rit brand Whitener and Brightener . Follow the package instructions. Both of these treatments are much gentler than bleaching. They are, in effect, temporary dyes. By reflecting blue light, they make the fabric appear to be whiter.
There is no cure for fabric that contains polyester and has been yellowed by bleach damage. Polyester cannot tolerate treatment with chlorine bleach.
(Please help support this web site. Thank you.)
Friday, July 13, 2007
We are planning to tie dye a blanket for the horse but having problems coming up with a REALLY neat tie dyeing pattern so can you please help us out?
Name: Tami
Message: My daughter is showing her horse at our County Fair and is enter in the costume contest, she has signed up for the Patriotic class. We are planning to tie dye a blanket for the horse but having problems coming up with a REALLY neat tie dyeing pattern so can you please help us out?
Check out the different designs at the following links:
"Tie Dyeing" in the Dye Forum Community of Dyers
Tie-dye Wiki (with instructions)
itiedye.com Tie-Dye Gallery (no instructions, but see the Wiki, above)
(Please help support this web site. Thank you.)
Message: My daughter is showing her horse at our County Fair and is enter in the costume contest, she has signed up for the Patriotic class. We are planning to tie dye a blanket for the horse but having problems coming up with a REALLY neat tie dyeing pattern so can you please help us out?
Check out the different designs at the following links:
"Tie Dyeing" in the Dye Forum Community of Dyers
Tie-dye Wiki (with instructions)
itiedye.com Tie-Dye Gallery (no instructions, but see the Wiki, above)
(Please help support this web site. Thank you.)
Thursday, July 12, 2007
My deep, intense colors came out pastel in the wash-out!
Name: Michelle
Message: I searched, this situation not covered.
My deep, intense colors came out pastel in the wash-out!
I used procion dyes @ the correct temp, used soda ash, pH tested my soda ash, 100% cotton fabric, dye stayed on for 12 hours in a hot room (I live in AZ). My urea is a few years old, but does not smell bad in its dry form.
However, I have been using a dye premix (mix up urea, kelp, and water, so you can just pour off & add dye later) and the premix I used for this batch was a few weeks old, and sat in a very hot room. I noticed when I was washing out the shirts they smelled of amonia. Do you think that's what did it?
I made a fresh batch of premix and it doesn't smell...I've never had this happen before and just did a dozen shirts that came out like this. Do you think they'll be ok after they're washed? I want to know if they are ok to sell, or if I should trash them.
Thank you so much! :) Love the site :)
Yes, there was one case in which failure of fiber reactive dye turned out to be due to ammonia in the dye mixtures, from old urea. The reason for the problem is that ammonia increases the pH of the dye mixture, like soda ash does, and the Procion MX dye lasts for only an hour or so after it is added to a high-pH mixture. Unless you were working very quickly, it's likely that your dye was partially used up before you got it onto your fabric.
If that's the explanation, this last batch should have turned out all right. Did it? There are other possibilities, all of which are less likely, but which sometimes happen, almost as freak accidents. For example, in a couple of cases, people have thought they mixed up soda ash, but accidentally used urea instead, which, as you can imagine, does not do a great deal of good when used as a presoak. Obviously this was not the case for you, since you pH-tested your soda ash. (Good move!)
If the shirts come out pale, don't trash them, overdye them. Sometimes you can get much more interesting results than you would have with a single layer of dye.
(Please help support this web site. Thank you.)
Message: I searched, this situation not covered.
My deep, intense colors came out pastel in the wash-out!
I used procion dyes @ the correct temp, used soda ash, pH tested my soda ash, 100% cotton fabric, dye stayed on for 12 hours in a hot room (I live in AZ). My urea is a few years old, but does not smell bad in its dry form.
However, I have been using a dye premix (mix up urea, kelp, and water, so you can just pour off & add dye later) and the premix I used for this batch was a few weeks old, and sat in a very hot room. I noticed when I was washing out the shirts they smelled of amonia. Do you think that's what did it?
I made a fresh batch of premix and it doesn't smell...I've never had this happen before and just did a dozen shirts that came out like this. Do you think they'll be ok after they're washed? I want to know if they are ok to sell, or if I should trash them.
Thank you so much! :) Love the site :)
Yes, there was one case in which failure of fiber reactive dye turned out to be due to ammonia in the dye mixtures, from old urea. The reason for the problem is that ammonia increases the pH of the dye mixture, like soda ash does, and the Procion MX dye lasts for only an hour or so after it is added to a high-pH mixture. Unless you were working very quickly, it's likely that your dye was partially used up before you got it onto your fabric.
If that's the explanation, this last batch should have turned out all right. Did it? There are other possibilities, all of which are less likely, but which sometimes happen, almost as freak accidents. For example, in a couple of cases, people have thought they mixed up soda ash, but accidentally used urea instead, which, as you can imagine, does not do a great deal of good when used as a presoak. Obviously this was not the case for you, since you pH-tested your soda ash. (Good move!)
If the shirts come out pale, don't trash them, overdye them. Sometimes you can get much more interesting results than you would have with a single layer of dye.
(Please help support this web site. Thank you.)
Wednesday, July 11, 2007
I already used all purpose dye - some of these were for children. Is that a serious problem?
Name: Shirl
Message: I already used all purpose dye - some of these were for children. Is that a serious problem? Thanks!
I don't think that all-purpose dye is unsafe, but the results will probably not last as long in the laundry as you like. If you used cool water to dye with all-purpose dye, the dye may wash out immediately! All-purpose dye is a hot water dye and works best when the clothing is heated in the dyebath for half an hour or longer at a temperature just below boiling.
If you give the dyed items as gifts, be sure to include a label with care instructions: "hand wash separately in cool water". Hot water will remove all-purpose dye more quickly than cool water will. Washing separately will prevent laundry disasters in which the all-purpose dye bleeds onto other clothing and ruins it. Never sell a garment whose dye is not completely fixed.
To improve washfastness, you can use a commercial dye fixative, such as Retayne. You will probably have to mail-order it, but your local fabric store might carry it. You cannot fix all-purpose dye with simpler chemicals such as salt, soda ash, or vinegar. See "Commercial Dye Fixatives".
In the future, if you are dyeing cotton clothing, you will want to use fiber reactive dye. Good tie-dye kits, made by Jacquard Products, Rainbow Rock, Tulip, Dritz, and Dylon, can sometimes be found in your local crafts store, or, for a much lower price per shirt and a better range of color choices, you can buy your dyes by mail-order. Look for Procion MX dyes. I maintain a list of places to buy dyeing supplies around the world.
(Please help support this web site. Thank you.)
Message: I already used all purpose dye - some of these were for children. Is that a serious problem? Thanks!
I don't think that all-purpose dye is unsafe, but the results will probably not last as long in the laundry as you like. If you used cool water to dye with all-purpose dye, the dye may wash out immediately! All-purpose dye is a hot water dye and works best when the clothing is heated in the dyebath for half an hour or longer at a temperature just below boiling.
If you give the dyed items as gifts, be sure to include a label with care instructions: "hand wash separately in cool water". Hot water will remove all-purpose dye more quickly than cool water will. Washing separately will prevent laundry disasters in which the all-purpose dye bleeds onto other clothing and ruins it. Never sell a garment whose dye is not completely fixed.
To improve washfastness, you can use a commercial dye fixative, such as Retayne. You will probably have to mail-order it, but your local fabric store might carry it. You cannot fix all-purpose dye with simpler chemicals such as salt, soda ash, or vinegar. See "Commercial Dye Fixatives".
In the future, if you are dyeing cotton clothing, you will want to use fiber reactive dye. Good tie-dye kits, made by Jacquard Products, Rainbow Rock, Tulip, Dritz, and Dylon, can sometimes be found in your local crafts store, or, for a much lower price per shirt and a better range of color choices, you can buy your dyes by mail-order. Look for Procion MX dyes. I maintain a list of places to buy dyeing supplies around the world.
(Please help support this web site. Thank you.)
Tuesday, July 10, 2007
I am trying to tie dye foldover elastic but the colors just wash away. So far I have used Procion MX and Rit dye products but neither have worked. What am I doing wrong?
Name: Susan
Message: I am trying to tie dye foldover elastic but the colors just wash away. So far I have used Procion MX and Rit dye products but neither have worked. What am I doing wrong?
You have to match both your dye type and your dyeing recipe to the fiber content of whatever you're dyeing. Do you know the fiber content of your fold over elastic?
There is some fold over elastic being sold online whose fiber content is 81% nylon and 19% Lycra spandex. See the following page for more information on dyeing spandex and spandex blends: "How to Dye Spandex (also known as Lycra® or elastane)"
Don't even try to dye the spandex in the fiber blend; just concentrate on the nylon. Nylon can be dyed with acid dyes, and it can also be colored with fabric paints whose manufacturers say they will work well on synthetic fibers, such as Dye-na-flow or Lumiere fabric paints. See "About Acid Dyes" and "Fabric Paints: a different way to color fibers".
Rit dye, as an all-purpose dye, contains a kind of acid dye, as well as another dye which will probably just wash out of nylon. However, acid dyes cannot work unless the pH of your dyebath is altered by adding a mild acid such as vinegar. If you use Rit dye on your elastic again, try adding 5 teaspoons of distilled white vinegar per quart of water to a hot dyebath (in which you must immerse your elastic for half an hour or so). Procion MX dyes can also be used as acid dye, if you substitute vinegar or citric acid for the soda ash usually used with Procion MX dyes, and, again, use heat. Procion MX dye costs much less than an equivalent amount of Rit dye powder, and is superior for use on cotton. See "Fiber reactive dyes on protein fibers", especially the links to recipes that you can use. Acid dyes will not work at room temperature, but instead should be used at the highest temperature that can be tolerated by your fabric. Unfortunately, spandex is very heat-sensitive, so you will have to compromise between the higher water temperature which is more effective for dyeing nylon, and the temperature which is safe for use on spandex.
Most fabric paints require the use of high heat to set the paint, by melting the binder in the paint into the fiber. This would damage the spandex in your elastic. If you choose to use fabric paint, I recommend that you consider ordering some Jacquard Airfix to add to your paint so that it does not require heat-setting. You can find sources for Jacquard products on their web site.
If your elastic contains polyester, rather than nylon, you will not be able to use dyes, as polyester dye requires extended boiling in the dyebath; this much heat will damage your spandex. In that case, a good fabric paint would be your only option for coloring your elastic.
(Please help support this web site. Thank you.)
Message: I am trying to tie dye foldover elastic but the colors just wash away. So far I have used Procion MX and Rit dye products but neither have worked. What am I doing wrong?
You have to match both your dye type and your dyeing recipe to the fiber content of whatever you're dyeing. Do you know the fiber content of your fold over elastic?
There is some fold over elastic being sold online whose fiber content is 81% nylon and 19% Lycra spandex. See the following page for more information on dyeing spandex and spandex blends: "How to Dye Spandex (also known as Lycra® or elastane)"
Don't even try to dye the spandex in the fiber blend; just concentrate on the nylon. Nylon can be dyed with acid dyes, and it can also be colored with fabric paints whose manufacturers say they will work well on synthetic fibers, such as Dye-na-flow or Lumiere fabric paints. See "About Acid Dyes" and "Fabric Paints: a different way to color fibers".
Rit dye, as an all-purpose dye, contains a kind of acid dye, as well as another dye which will probably just wash out of nylon. However, acid dyes cannot work unless the pH of your dyebath is altered by adding a mild acid such as vinegar. If you use Rit dye on your elastic again, try adding 5 teaspoons of distilled white vinegar per quart of water to a hot dyebath (in which you must immerse your elastic for half an hour or so). Procion MX dyes can also be used as acid dye, if you substitute vinegar or citric acid for the soda ash usually used with Procion MX dyes, and, again, use heat. Procion MX dye costs much less than an equivalent amount of Rit dye powder, and is superior for use on cotton. See "Fiber reactive dyes on protein fibers", especially the links to recipes that you can use. Acid dyes will not work at room temperature, but instead should be used at the highest temperature that can be tolerated by your fabric. Unfortunately, spandex is very heat-sensitive, so you will have to compromise between the higher water temperature which is more effective for dyeing nylon, and the temperature which is safe for use on spandex.
Most fabric paints require the use of high heat to set the paint, by melting the binder in the paint into the fiber. This would damage the spandex in your elastic. If you choose to use fabric paint, I recommend that you consider ordering some Jacquard Airfix to add to your paint so that it does not require heat-setting. You can find sources for Jacquard products on their web site.
If your elastic contains polyester, rather than nylon, you will not be able to use dyes, as polyester dye requires extended boiling in the dyebath; this much heat will damage your spandex. In that case, a good fabric paint would be your only option for coloring your elastic.
(Please help support this web site. Thank you.)
Thursday, July 05, 2007
Can you please tell me if it's possible to dye black or gray clothing, & if so, how?
Can you please tell me if it's possible to dye black or gray
clothing & if so, how? I couldn't find the answer on your site. Thank
you.
Are you asking about dyeing clothing that is already black or dark in color to a brighter or lighter color, or are you asking about what is the best way to dye clothing to make it black or grey?
Dyeing clothing that is already black or dark in color to a brighter or lighter color.
Dye is transparent. This means that if you dye anything that already has a color, the result will be the new color added on to the old one. Overdyeing black results in nothing other than black.
In order to change black clothing to a lighter color, you must discharge, or remove, the dye that is already in it. This is not always possible. Some dyes will resist any discharge chemical, retaining their color even after the fabric itself has been damaged.
Synthetic fibers of any sort, as well as protein fibers such as silk or wool, must never be subjected to hypochlorite, which is the active ingredient in ordinary household chlorine bleach. An alternative to bleach, Rit Color Remover, which is based on sodium dithionite (also known as sodium hydrosulfite), is less damaging to protein or synthetic fibers; it works well on some dyes, but not on other dyes. You will need to buy several boxes for one washing machine load; in the US, you can find Rit Color Remover at most fabric stores as well as many grocery stores and pharmacies.
If you use bleach to remove the color from 100% cotton garments, you should neutralize the bleach, after rinsing. Do not use vinegar for this purpose. See the instructions on the following page from my FAQ:
How can I neutralize the damaging effects of chlorine bleach?
http://www.pburch.net/dyeing/FAQ/neutralizingdischarge.shtml
You do not need to chemically neutralize after using Rit Color Remover.
After you have used chlorine bleach or Rit Color Remover to remove the dye from your fabric, if that turns out to be possible, you will probably find that the polyester thread used to stitch the garment together remains the original color. There is nothing that can be done to solve this problem, if it occurs.
Bleached fabric can be dyed like any other fabric of its color. Any remaining color will be incorporated into your final color results. You should choose your dye carefully based on the fiber content of your clothing; see "About Dyes", at http://www.pburch.net/dyeing/aboutdyes.shtml . Cool water fiber reactive dyes, such as Procion MX dye, will give better results on cotton than you will be able to obtain with all-purpose dyes, such as Rit dye.
(For information on how you can support this resource, please see "About This Site".)
Are you asking about dyeing clothing that is already black or dark in color to a brighter or lighter color, or are you asking about what is the best way to dye clothing to make it black or grey?
Dyeing clothing that is already black or dark in color to a brighter or lighter color.
Dye is transparent. This means that if you dye anything that already has a color, the result will be the new color added on to the old one. Overdyeing black results in nothing other than black.
In order to change black clothing to a lighter color, you must discharge, or remove, the dye that is already in it. This is not always possible. Some dyes will resist any discharge chemical, retaining their color even after the fabric itself has been damaged.
Synthetic fibers of any sort, as well as protein fibers such as silk or wool, must never be subjected to hypochlorite, which is the active ingredient in ordinary household chlorine bleach. An alternative to bleach, Rit Color Remover, which is based on sodium dithionite (also known as sodium hydrosulfite), is less damaging to protein or synthetic fibers; it works well on some dyes, but not on other dyes. You will need to buy several boxes for one washing machine load; in the US, you can find Rit Color Remover at most fabric stores as well as many grocery stores and pharmacies.
If you use bleach to remove the color from 100% cotton garments, you should neutralize the bleach, after rinsing. Do not use vinegar for this purpose. See the instructions on the following page from my FAQ:
How can I neutralize the damaging effects of chlorine bleach?
http://www.pburch.net/dyeing/FAQ/neutralizingdischarge.shtml
You do not need to chemically neutralize after using Rit Color Remover.
After you have used chlorine bleach or Rit Color Remover to remove the dye from your fabric, if that turns out to be possible, you will probably find that the polyester thread used to stitch the garment together remains the original color. There is nothing that can be done to solve this problem, if it occurs.
Bleached fabric can be dyed like any other fabric of its color. Any remaining color will be incorporated into your final color results. You should choose your dye carefully based on the fiber content of your clothing; see "About Dyes", at http://www.pburch.net/dyeing/aboutdyes.shtml . Cool water fiber reactive dyes, such as Procion MX dye, will give better results on cotton than you will be able to obtain with all-purpose dyes, such as Rit dye.
(For information on how you can support this resource, please see "About This Site".)
Wednesday, July 04, 2007
What is the purpose of salt in the dye mix?
Name: m corder
Message: What is the purpose of salt in the dye mix? The recipe I am using calls for urea, salt and cold water dye (about 30 cups urea, 7 cups dye, and 1 cup salt per vat; then one cup of this mixture is mixed with 2 quarts of water for the dye used that day).
There is a page about salt in the FAQ on my site, "Do I need to use salt, in dyeing?", which includes the following:
"Contrary to some old wives' tales, salt is not a dye fixative and does nothing to make dye more permanent; however, it aids in the dyeing process by helping to drive the dye onto the fiber, out of solution, so that it is in the right place for any bonding to the fiber to occur."
The way salt works is by surrounding the fiber, which in water has a negative electrical charge. Most dyes also have a negative electrical charge (the two exceptions being basic dyes, which have a positive charge, and vat dyes, which are neutral). The salt stops the negative charge of the fiber from repelling the negative charge of the dye.
When reactive dyes are applied directly to the fiber in small quantities of water, as in the cases of tie-dyeing and dye painting, salt is not required because the dye is placed right next to the fiber, instead of floating around in a large quantity of water, as in vat dyeing. The salt is very important in dyeing with the large amount of water that is required for smooth, eve, solid-colored dyeing.
Your recipe is one that is unfamiliar to me. I have not heard of using such large amounts of urea, and I have not seen recipes for vat dyeing with cool water dyes that required any urea at all, except possibly one tablespoon per cup of the water initially used to dissolve the dye. A typical recipe for dyeing five pounds of cotton with cool water fiber reactive dye calls for using two and a half ounces of dye powder, twenty cups of salt, and two and a half cups of soda ash, in twenty gallons of water.
(Please help support this web site. Thank you.)
Message: What is the purpose of salt in the dye mix? The recipe I am using calls for urea, salt and cold water dye (about 30 cups urea, 7 cups dye, and 1 cup salt per vat; then one cup of this mixture is mixed with 2 quarts of water for the dye used that day).
There is a page about salt in the FAQ on my site, "Do I need to use salt, in dyeing?", which includes the following:
"Contrary to some old wives' tales, salt is not a dye fixative and does nothing to make dye more permanent; however, it aids in the dyeing process by helping to drive the dye onto the fiber, out of solution, so that it is in the right place for any bonding to the fiber to occur."
The way salt works is by surrounding the fiber, which in water has a negative electrical charge. Most dyes also have a negative electrical charge (the two exceptions being basic dyes, which have a positive charge, and vat dyes, which are neutral). The salt stops the negative charge of the fiber from repelling the negative charge of the dye.
When reactive dyes are applied directly to the fiber in small quantities of water, as in the cases of tie-dyeing and dye painting, salt is not required because the dye is placed right next to the fiber, instead of floating around in a large quantity of water, as in vat dyeing. The salt is very important in dyeing with the large amount of water that is required for smooth, eve, solid-colored dyeing.
Your recipe is one that is unfamiliar to me. I have not heard of using such large amounts of urea, and I have not seen recipes for vat dyeing with cool water dyes that required any urea at all, except possibly one tablespoon per cup of the water initially used to dissolve the dye. A typical recipe for dyeing five pounds of cotton with cool water fiber reactive dye calls for using two and a half ounces of dye powder, twenty cups of salt, and two and a half cups of soda ash, in twenty gallons of water.
(Please help support this web site. Thank you.)
Tuesday, July 03, 2007
I used to have a recipe that used resist salts L, calgon, and urea mixed with soda ash and bicarb. The results are super but I have lost the recipe do you have it?
Message: I used to have a recipe that used resist salts L, calgon,
and urea mixed with soda ash and bicarb. The results are super but I have lost
the recipe do you have
it?
What kind of dye are you using? From the names of the other chemicals, it seems likely that it's either Procion MX dye, or Procion H dye. These two types of Procion dye are chemically similar, but very different in the amount of heat needed to fix the dye to the fabric. Procion H must be fixed by steam or in a hot dyebath, while Procion MX dyes are used at room temperature.
Resist salt L is another name for the chemical sodium m-nitrobenzene sulfonate, also known as Ludigol (see "Why do some dye recipes call for Ludigol?"). It is important when steaming or microwaving Procion dyes, which otherwise may be chemically reduced at high temperatures, so it should always be used when you are working with Procion H dyes, which require heat-setting. PRO Chemical & Dye recommends its use also in areas with high levels of air pollution. Otherwise, there is no need to use it with dyes that are fixed at room temperature, such as Procion MX dye.
Soda ash (sodium carbonate) may be mixed with sodium bicarbonate when one is painting with dyes that will be steam set, or sodium bicarbonate can be substituted altogether, because bicarbonate does not encourage the reaction of the dye with the fiber very well at room temperature. Sodium bicarbonate turns to sodium carbonate at high temperatures, when it is baked or steamed. The less-than-optimal pH of soda ash mixed with bicarb (as opposed to soda ash alone) slows dye reactions a little at room temperature, but does not make them stop completely.
Calgon T is sodium hexametaphosphate, and is important for dyeing in areas where the water supply is "hard", which means that it contains calcium and/or magnesium salts. You can purchase it from dye suppliers under that name or, frequently, as Metaphos.
Other than the names used for your chemicals your recipe looks very much like these two recipes provided by ProChem:
Direct Application using PRO H-Reactive Dyes
Direct Application using PRO MX Reactive Dyes
where "PRO Dye Activator" is soda ash, and "PRO Chem Flakes" is Ludigol,
as well as this recipe by Jacquard Products:
Procion H Concentrate Fiber Reactive Dyes Instructions.
(Please help support this web site. Thank you.)
What kind of dye are you using? From the names of the other chemicals, it seems likely that it's either Procion MX dye, or Procion H dye. These two types of Procion dye are chemically similar, but very different in the amount of heat needed to fix the dye to the fabric. Procion H must be fixed by steam or in a hot dyebath, while Procion MX dyes are used at room temperature.
Resist salt L is another name for the chemical sodium m-nitrobenzene sulfonate, also known as Ludigol (see "Why do some dye recipes call for Ludigol?"). It is important when steaming or microwaving Procion dyes, which otherwise may be chemically reduced at high temperatures, so it should always be used when you are working with Procion H dyes, which require heat-setting. PRO Chemical & Dye recommends its use also in areas with high levels of air pollution. Otherwise, there is no need to use it with dyes that are fixed at room temperature, such as Procion MX dye.
Soda ash (sodium carbonate) may be mixed with sodium bicarbonate when one is painting with dyes that will be steam set, or sodium bicarbonate can be substituted altogether, because bicarbonate does not encourage the reaction of the dye with the fiber very well at room temperature. Sodium bicarbonate turns to sodium carbonate at high temperatures, when it is baked or steamed. The less-than-optimal pH of soda ash mixed with bicarb (as opposed to soda ash alone) slows dye reactions a little at room temperature, but does not make them stop completely.
Calgon T is sodium hexametaphosphate, and is important for dyeing in areas where the water supply is "hard", which means that it contains calcium and/or magnesium salts. You can purchase it from dye suppliers under that name or, frequently, as Metaphos.
Other than the names used for your chemicals your recipe looks very much like these two recipes provided by ProChem:
Direct Application using PRO H-Reactive Dyes
Direct Application using PRO MX Reactive Dyes
where "PRO Dye Activator" is soda ash, and "PRO Chem Flakes" is Ludigol,
as well as this recipe by Jacquard Products:
Procion H Concentrate Fiber Reactive Dyes Instructions.
(Please help support this web site. Thank you.)
Monday, July 02, 2007
What I am looking for is that amazing in-your-face clear blue that shows up in Nancy Crow's quilts. Any ideas?
Name: judy
Message: Dear Paula, Thank you for your amazing site. A friend of mine came for a dyeing day and brought a jar of Dharma's Electric Blue, PR23A. I was wondering if there is a ProChemical equivalent, as I will be up at ProChem shortly. Actually, what I am looking for is that amazing in-your-face clear blue that shows up in Nancy Crow's quilts. Any ideas?
There is no perfect equivalent for any of the proprietary mixtures that are prepared by any of the dye suppliers. The exact contents of Dharma's "Electric Blue" are a trade secret, unlikely to be perfectly identical to any mixture at another dye seller. The only dyes for which the identical dye is sold at other companies are the ones that are either pure, unmixed dyes, or the 'manufacturer's mixes' which are premixed by the dye manufacturers before they are sold to Dharma or Prochem. The unmixed, single hue colors are listed on the main chart on my page, "Which Procion MX colors are pure, and which mixtures?", at http://www.pburch.net/dyeing/FAQ/pureMXcolors.shtml . The manufacturer's mixtures have MX codes but are not in the top chart; many are listed in the second table, at the bottom of that page.
It looks to me as though a good substitute for that blue might be a mixture of equal parts of cerulean blue, blue MX-G, which ProChem calls intense blue, plus turquoise MX-G, which all suppliers of Procion MX type dyes sell under the same name. This is only a guess, however, judged merely from the color chip on their web page. You will have to use trial and error to determine whether this is a good color or not. Of course, ProChem has many premixed blues. Try looking at their color charts for a dye color that seems likely to be what you want. A lot of quilters like to use ProChem's Brightest Blue 404. I never have used it, since it's a mixture. You might want to try it, as well.
I strongly recommend that you buy some of each of the "pure" unmixed blues, including blue MX-R ("sky blue" at Dharma, "basic blue" at ProChem), blue MX-G ("cerulean blue" at Dharma, "intense blue" at ProChem), and turquoise MX-G ("Turquoise" at both suppliers), as well as one or more of the navies, such as blue MX-2G ("cobalt blue" at Dharma, "mixing blue" at ProChem). The pure unmixed hues give the brightest possible results when mixing colors, so it's good to have them all on hand and to learn their properties, if you haven't already. The brightest true blue in the Procion MX line may be that be obtained by mixing turquoise MX-G with a small amount of fuchsia (red MX-8B), though red MX-5B is a little better behaved with respect to making colored halos when the dye is applied directly to he fabric.
Please let me know your thoughts about the colors that you end up buying!
(Please help support this web site. Thank you.)
Message: Dear Paula, Thank you for your amazing site. A friend of mine came for a dyeing day and brought a jar of Dharma's Electric Blue, PR23A. I was wondering if there is a ProChemical equivalent, as I will be up at ProChem shortly. Actually, what I am looking for is that amazing in-your-face clear blue that shows up in Nancy Crow's quilts. Any ideas?
There is no perfect equivalent for any of the proprietary mixtures that are prepared by any of the dye suppliers. The exact contents of Dharma's "Electric Blue" are a trade secret, unlikely to be perfectly identical to any mixture at another dye seller. The only dyes for which the identical dye is sold at other companies are the ones that are either pure, unmixed dyes, or the 'manufacturer's mixes' which are premixed by the dye manufacturers before they are sold to Dharma or Prochem. The unmixed, single hue colors are listed on the main chart on my page, "Which Procion MX colors are pure, and which mixtures?", at http://www.pburch.net/dyeing/FAQ/pureMXcolors.shtml . The manufacturer's mixtures have MX codes but are not in the top chart; many are listed in the second table, at the bottom of that page.
It looks to me as though a good substitute for that blue might be a mixture of equal parts of cerulean blue, blue MX-G, which ProChem calls intense blue, plus turquoise MX-G, which all suppliers of Procion MX type dyes sell under the same name. This is only a guess, however, judged merely from the color chip on their web page. You will have to use trial and error to determine whether this is a good color or not. Of course, ProChem has many premixed blues. Try looking at their color charts for a dye color that seems likely to be what you want. A lot of quilters like to use ProChem's Brightest Blue 404. I never have used it, since it's a mixture. You might want to try it, as well.
I strongly recommend that you buy some of each of the "pure" unmixed blues, including blue MX-R ("sky blue" at Dharma, "basic blue" at ProChem), blue MX-G ("cerulean blue" at Dharma, "intense blue" at ProChem), and turquoise MX-G ("Turquoise" at both suppliers), as well as one or more of the navies, such as blue MX-2G ("cobalt blue" at Dharma, "mixing blue" at ProChem). The pure unmixed hues give the brightest possible results when mixing colors, so it's good to have them all on hand and to learn their properties, if you haven't already. The brightest true blue in the Procion MX line may be that be obtained by mixing turquoise MX-G with a small amount of fuchsia (red MX-8B), though red MX-5B is a little better behaved with respect to making colored halos when the dye is applied directly to he fabric.
Please let me know your thoughts about the colors that you end up buying!
(Please help support this web site. Thank you.)
Sunday, July 01, 2007
what dye would you recommend for the following garment: South American made jumpsuit with the following "espanol" materials - 60% algodao, 30% poliester, 10% elastano.
Name: Katie
Message: Hello, I'm curious if you would consider dyeing an item for me and I will ship it to you and would pay you? If not, what dye would you recommend for the following garment: South American made jumpsuit with the following "espanol" materials - 60% algodao, 30% poliester, 10% elastano. This garment is unworn, the color is winter white, and has a large number of studs/rivets that have bled a greenish color onto the white. This is why I am looking to color it. Thank you for your time...I look forward to your reply.
I'm afraid that I can't take on that project myself, but you could find another dyer to do it, if you look at the 'Find a Custom Dyer' list on my website, under the 'More' item on the menubar. (Try this link.)
Algodao is cotton. Cotton is an easily dyeable fiber, as long as there is no surface finish such as stain resistance or permanent press to interfere with the dye.
Poliester is polyester. Unfortunately, polyester cannot be dyed except at high temperatures that would destroy your garment, due to its sensitive spandex contents. You will have to leave the polyester undyed. Your garment will end up only 60% as bright or intense in color as a 100% cotton garment would.
Elastano is elastane, or spandex, often known by the brand name Lycra. Spandex is very heat-sensitive. When dyeing a garment that is 10% spandex, we normally do not attempt to dye the spandex itself, because it is impractical to do so after the spandex has been woven or knitted into a fabric blend.
You must avoid the use of all-purpose dye, such as Rit dye, when dyeing this garment. All-purpose dye requires hot water, which should never be used on a garment that contains spandex.
The best dye to use would be a cool water fiber reactive dye, such as Procion MX dye. You can dye your jumpsuit a solid color in the washing machine, but the stains may show through. In fact, the copper in the studs which is the source of the problem may even interact with a dye to create a new color. I would recommend that you dye the jumpsuit in multiple colors, as in the technique known as low water immersion dyeing. This method of dyeing is very easy, and creates beautiful mixtures of colors which may help to disguise the problem with the reactive metal used in the garment.
Message: Hello, I'm curious if you would consider dyeing an item for me and I will ship it to you and would pay you? If not, what dye would you recommend for the following garment: South American made jumpsuit with the following "espanol" materials - 60% algodao, 30% poliester, 10% elastano. This garment is unworn, the color is winter white, and has a large number of studs/rivets that have bled a greenish color onto the white. This is why I am looking to color it. Thank you for your time...I look forward to your reply.
I'm afraid that I can't take on that project myself, but you could find another dyer to do it, if you look at the 'Find a Custom Dyer' list on my website, under the 'More' item on the menubar. (Try this link.)
Algodao is cotton. Cotton is an easily dyeable fiber, as long as there is no surface finish such as stain resistance or permanent press to interfere with the dye.
Poliester is polyester. Unfortunately, polyester cannot be dyed except at high temperatures that would destroy your garment, due to its sensitive spandex contents. You will have to leave the polyester undyed. Your garment will end up only 60% as bright or intense in color as a 100% cotton garment would.
Elastano is elastane, or spandex, often known by the brand name Lycra. Spandex is very heat-sensitive. When dyeing a garment that is 10% spandex, we normally do not attempt to dye the spandex itself, because it is impractical to do so after the spandex has been woven or knitted into a fabric blend.
You must avoid the use of all-purpose dye, such as Rit dye, when dyeing this garment. All-purpose dye requires hot water, which should never be used on a garment that contains spandex.
The best dye to use would be a cool water fiber reactive dye, such as Procion MX dye. You can dye your jumpsuit a solid color in the washing machine, but the stains may show through. In fact, the copper in the studs which is the source of the problem may even interact with a dye to create a new color. I would recommend that you dye the jumpsuit in multiple colors, as in the technique known as low water immersion dyeing. This method of dyeing is very easy, and creates beautiful mixtures of colors which may help to disguise the problem with the reactive metal used in the garment.
When you say dye the suit in multiple colors...do you mean putting
multiple colors into the washing machine ... or just one color and see how it
looks then try another? I would worry that multiple colors used together
would look greyish or putrid when it was all done? Is this really a
project that is so easy? If so, I'm willing to do it myself. thanks
so much
That's a very important point. No, you do not use multiple colors in the washing machine, as you're right, they will mix together. Instead, for multiple colors, use low water immersion dyeing, which involves just stuffing the garment into a bucket, tightly, and pouring dyes and then soda ash over it. You must use a fiber reactive dye, such as Procion MX dye, for this recipe. It's extremely easy, easier than any other dye method, because you don't have to stir or heat-set or anything like that. Check out this page of instructions:
How to Do Low Water Immersion Dyeing
The interesting thing is that even clashing colors work very well together in this technique, because they blend at the edges to make subtler colors. I like to use colors that mix well together, such as blue with yellow, which will produce a garment with blue, yellow, and green.
You'll get better results on 100% cotton than on your 60% cotton jumpsuit. It will be interesting it you put a cotton t-shirt in with the jumpsuit and dye them at the same time; you will end up with a much brighter though coordinating version of the same colors.
(Please help support this web site. Thank you.)
That's a very important point. No, you do not use multiple colors in the washing machine, as you're right, they will mix together. Instead, for multiple colors, use low water immersion dyeing, which involves just stuffing the garment into a bucket, tightly, and pouring dyes and then soda ash over it. You must use a fiber reactive dye, such as Procion MX dye, for this recipe. It's extremely easy, easier than any other dye method, because you don't have to stir or heat-set or anything like that. Check out this page of instructions:
How to Do Low Water Immersion Dyeing
The interesting thing is that even clashing colors work very well together in this technique, because they blend at the edges to make subtler colors. I like to use colors that mix well together, such as blue with yellow, which will produce a garment with blue, yellow, and green.
You'll get better results on 100% cotton than on your 60% cotton jumpsuit. It will be interesting it you put a cotton t-shirt in with the jumpsuit and dye them at the same time; you will end up with a much brighter though coordinating version of the same colors.
(Please help support this web site. Thank you.)
