« 2006 December | Main | 2006 October »
Wednesday, November 22, 2006
What is the purpose of "batching" of procion dyes?
Saturday, November 18, 2006
How can I measure the results of a science fair project to compare dyes at different pHs?
Friday, November 17, 2006
safe non-toxic dyes for dog toys
Thursday, November 16, 2006
Could we successfully dye the veil to match, by Saturday?
Wednesday, November 15, 2006
While I see how both cotton and polyester are to be died, what do you do in the case of an article being 65% polyester and 35% cotton?
Tuesday, November 14, 2006
mixing a true red with Procion MX dyes
Monday, November 13, 2006
problems with direct application of indigo dye
Sunday, November 12, 2006
I am thinking of purchasing a white 100% rayon velvet wedding gown w/the intentions of dyeing it blue. I've never dyed anything in my life. First of all is this possible?
Saturday, November 11, 2006
My batiked shirts never seem as vibrant as just tie-dyed shirts. Any suggestions?
Friday, November 10, 2006
Do I really need to use fixative with Dylon Cold Dye, or is that them trying to get me to buy more?
Saturday, November 04, 2006
How can I get my dyed towels and washcloths to quit bleeding out? I've washed and rinsed and soaked these articles well over 50 times and they still 'bleed'.
Wednesday, November 01, 2006
beautiful raindrop and malachite effects from salt and alcohol in silk painting
What is the purpose of "batching" of procion dyes?
What is the purpose of "batching" of procion dyes? Is it for heat
setting? or just to intensify the dyes color (uptake)? Am I mistaken to think
that it is only the soda ash that fixes the dye to/in the fiber? or does it take
some heat to do
so?
The "batching" is just the time allowed for the dye to react. A fiber reactive dye actually reacts chemically with the fiber to become one molecule. If you don't allow enough time, not all of the dye molecules will become connected to the fiber. All of the unreacted dye will just wash out, because it is not attached to the fiber. Only the dye molecules that have reacted with the fiber will stay.
The reaction will not proceed without both a high pH and a certain amount of warmth, plus at least a little moisture. The soda ash provides the high pH; almost any chemical that raises the pH enough will do the same job, but soda ash is the safest and easiest to use.
The reaction proceeds more quickly at higher temperatures. If more warmth is provided, less time is needed. Overnight at 70°F works, for Procion MX dyes, or a few hours at 90°F, or a few minutes at 120°F. Temperatures below 70°F are just too low and won't work very well no matter how long you leave it. Other types of fiber reactive dyes require a little more warmth.
(Please help support this web site. Thank you.)
The "batching" is just the time allowed for the dye to react. A fiber reactive dye actually reacts chemically with the fiber to become one molecule. If you don't allow enough time, not all of the dye molecules will become connected to the fiber. All of the unreacted dye will just wash out, because it is not attached to the fiber. Only the dye molecules that have reacted with the fiber will stay.
The reaction will not proceed without both a high pH and a certain amount of warmth, plus at least a little moisture. The soda ash provides the high pH; almost any chemical that raises the pH enough will do the same job, but soda ash is the safest and easiest to use.
The reaction proceeds more quickly at higher temperatures. If more warmth is provided, less time is needed. Overnight at 70°F works, for Procion MX dyes, or a few hours at 90°F, or a few minutes at 120°F. Temperatures below 70°F are just too low and won't work very well no matter how long you leave it. Other types of fiber reactive dyes require a little more warmth.
(Please help support this web site. Thank you.)
Saturday, November 18, 2006
How can I measure the results of a science fair project to compare dyes at different pHs?
Name: elliot
Message: hi, I'm a high school student and I am doing a science fair project on dyeing. I'm testing whether pH has an effect on the color shade. I'm going to be dyeing cotton fabric. The one problem with the experiment is that I don't know exactly how to measure the shade, I was planning on just getting the paint color samples and matching them to those shades. I was wondering if you had any sugestions or if you thought that was the best way.
What kind of dye are you going to be using? It is very important that you be able to specify exactly what dye you use, in your project. If you buy the right kind of dye, you can give the scientific name and even the chemical structure of the dye. However, if you buy Rit brand all-purpose dye, you will not be able to say anything at all about the specific dye you are using, because they keep it a secret from their customers. I strongly recommend against using all-purpose dye in science projects.
If you use a fiber reactive dye, you will know a lot more about your dye, and your project will be much more scientific. Often local crafts stores will carry Tulip brand tie-dye kits, which contain an excellent form of fiber reactive dye called Procion MX dye. Some fabrics stores also carry Dylon Permanent brand dye; the dye ingredients for many of the Dylon Permanent dye colors are listed here: "Dylon dyes", posted 9/27/2006. If you have time, you can mail-order good Procion MX dyes through Amazon (see colors and names for ordering Procion MX dyes through Amazon) or from any of the various dye suppliers list on my chart of Sources for Dyeing Supplies Around the World.
Cotton is a good choice; be careful to use cotton that does not have any suface finishes, such as stain-resistance or permanent press, and prewash it in hot water before you use it. It's important to make a clear description in your project results of exactly what fiber type you are dyeing. For most dyes, the best fabrics to use are 100% natural fibers, such as cotton or wool. (Superwash® treatment of wool is okay.) Some dyes work well on one fiber, others on a different fiber: see "About Dyes" on my website. It can make for a better project to compare the effectiveness of one dye on two very different fibers, such as cotton (which is cellulose) and wool (which is a protein). You will see that wool takes dye well at an acid pH, while cotton takes dye only at a basic (alkaline) pH.
When my son did a science fair project in middle school comparing the effects of pH on a specific dichlorotriazine dye, he used Procion Red MX-5B (also known as Color Index Reactive Red 2: see the different names on my chart of "Which Procion MX colors are pure, and which mixtures?"). He compared the action of this dye at a specific room temperature (don't forget to write down your own room temperature, and be sure your room is at least 70°F or 21°C or above for your dye reactions), at various pHs. He tested his dyebath pHs with pH paper before adding the same amounts of the same dye concentrates to each one.
I helped him with the measurement step after he had washed the fabric samples in cool and then hot water (I recommend a net lingerie bag for easily washing all of the samples at once in the washing machine) and allowed them to dry. Since you are in high school, you will probably be able to manage it by yourself. He used an ordinary digital computer scanner to scan in all of his different samples as one image, arranged on the scanner at one time. It is important to scan them together, because one scan may have a different overall color cast or brightness than another; scanning them in one pass helps to maintain consistency. We used Adobe Photoshop Elements 2.0. On the version of the program I have available now, Adobe Photoshop Elements 3.0 for Mac, the function we used is under the "Window" menu; click on "Histogram" to see a graph of the color intensity. We selected the channel labeled "Luminosity", and, after selecting a representation section of a color swatch with the Rectangular Marquée tool, wrote down what appeared as the mean value, as well as noting down the standard deviation. Now, the luminosity has a maximum value of 256. What my son was interested in was not the luminosity, but rather the intensity of dye color. To get the value for dye intesity, he subtracted the mean luminosity value from the number 256. This gave a number for dye intensity, in arbitrary units. He prepared graphs comparing the dye intensity value obtained in this way, to show the effects of various pHs. The Y-axis of his graphs were labeled "Dye Intensity (arbitrary units)" and had a maximum of 256, or perhaps he divided the resulting numbers by 256 to get a percent maximum dye intensity. Either would work.
Paint chips are not ideal for comparison because there is no numerical indication of the intensity of the color. Pantone color sets have the same problem but have the advantage of representing a widely-accepted standard; unfortunately their cost is quite prohibitive. The human eye is actually extremely accurate at comparing two adjacent colors, as long as you do not attempt to use color memory to compare two colors that are not side by side. What would be best, if you do not have access to a program like Adobe Photoshop Elements, would be to get a more scientific to use to visually compare your different results. For example, look at the grayscale charts on the following page:
http://www.oceanlight.com/html/about_color.html . As described there, one of the grayscale charts shows "0% (pure black) through 10, 20, 30, 40, 60, 70, 80 and 90% to 100% (pure white)". This is just what you need, if you cannot use a scanner or other optical device to digitize your results. If you use only one color of dye (be sure it's a single dye color, not a pre-mixed dye color containing more than one color), then you should be able to match the color intensity roughly, by eye, to the percent color intensity shown in the grayscale, allowing you to match a graphable number to each of your dyed test swatches of fabric.
Good luck with your project.
(Please help support this web site. Thank you.)
Message: hi, I'm a high school student and I am doing a science fair project on dyeing. I'm testing whether pH has an effect on the color shade. I'm going to be dyeing cotton fabric. The one problem with the experiment is that I don't know exactly how to measure the shade, I was planning on just getting the paint color samples and matching them to those shades. I was wondering if you had any sugestions or if you thought that was the best way.
What kind of dye are you going to be using? It is very important that you be able to specify exactly what dye you use, in your project. If you buy the right kind of dye, you can give the scientific name and even the chemical structure of the dye. However, if you buy Rit brand all-purpose dye, you will not be able to say anything at all about the specific dye you are using, because they keep it a secret from their customers. I strongly recommend against using all-purpose dye in science projects.
If you use a fiber reactive dye, you will know a lot more about your dye, and your project will be much more scientific. Often local crafts stores will carry Tulip brand tie-dye kits, which contain an excellent form of fiber reactive dye called Procion MX dye. Some fabrics stores also carry Dylon Permanent brand dye; the dye ingredients for many of the Dylon Permanent dye colors are listed here: "Dylon dyes", posted 9/27/2006. If you have time, you can mail-order good Procion MX dyes through Amazon (see colors and names for ordering Procion MX dyes through Amazon) or from any of the various dye suppliers list on my chart of Sources for Dyeing Supplies Around the World.
Cotton is a good choice; be careful to use cotton that does not have any suface finishes, such as stain-resistance or permanent press, and prewash it in hot water before you use it. It's important to make a clear description in your project results of exactly what fiber type you are dyeing. For most dyes, the best fabrics to use are 100% natural fibers, such as cotton or wool. (Superwash® treatment of wool is okay.) Some dyes work well on one fiber, others on a different fiber: see "About Dyes" on my website. It can make for a better project to compare the effectiveness of one dye on two very different fibers, such as cotton (which is cellulose) and wool (which is a protein). You will see that wool takes dye well at an acid pH, while cotton takes dye only at a basic (alkaline) pH.
When my son did a science fair project in middle school comparing the effects of pH on a specific dichlorotriazine dye, he used Procion Red MX-5B (also known as Color Index Reactive Red 2: see the different names on my chart of "Which Procion MX colors are pure, and which mixtures?"). He compared the action of this dye at a specific room temperature (don't forget to write down your own room temperature, and be sure your room is at least 70°F or 21°C or above for your dye reactions), at various pHs. He tested his dyebath pHs with pH paper before adding the same amounts of the same dye concentrates to each one.
I helped him with the measurement step after he had washed the fabric samples in cool and then hot water (I recommend a net lingerie bag for easily washing all of the samples at once in the washing machine) and allowed them to dry. Since you are in high school, you will probably be able to manage it by yourself. He used an ordinary digital computer scanner to scan in all of his different samples as one image, arranged on the scanner at one time. It is important to scan them together, because one scan may have a different overall color cast or brightness than another; scanning them in one pass helps to maintain consistency. We used Adobe Photoshop Elements 2.0. On the version of the program I have available now, Adobe Photoshop Elements 3.0 for Mac, the function we used is under the "Window" menu; click on "Histogram" to see a graph of the color intensity. We selected the channel labeled "Luminosity", and, after selecting a representation section of a color swatch with the Rectangular Marquée tool, wrote down what appeared as the mean value, as well as noting down the standard deviation. Now, the luminosity has a maximum value of 256. What my son was interested in was not the luminosity, but rather the intensity of dye color. To get the value for dye intesity, he subtracted the mean luminosity value from the number 256. This gave a number for dye intensity, in arbitrary units. He prepared graphs comparing the dye intensity value obtained in this way, to show the effects of various pHs. The Y-axis of his graphs were labeled "Dye Intensity (arbitrary units)" and had a maximum of 256, or perhaps he divided the resulting numbers by 256 to get a percent maximum dye intensity. Either would work.
Paint chips are not ideal for comparison because there is no numerical indication of the intensity of the color. Pantone color sets have the same problem but have the advantage of representing a widely-accepted standard; unfortunately their cost is quite prohibitive. The human eye is actually extremely accurate at comparing two adjacent colors, as long as you do not attempt to use color memory to compare two colors that are not side by side. What would be best, if you do not have access to a program like Adobe Photoshop Elements, would be to get a more scientific to use to visually compare your different results. For example, look at the grayscale charts on the following page:
http://www.oceanlight.com/html/about_color.html . As described there, one of the grayscale charts shows "0% (pure black) through 10, 20, 30, 40, 60, 70, 80 and 90% to 100% (pure white)". This is just what you need, if you cannot use a scanner or other optical device to digitize your results. If you use only one color of dye (be sure it's a single dye color, not a pre-mixed dye color containing more than one color), then you should be able to match the color intensity roughly, by eye, to the percent color intensity shown in the grayscale, allowing you to match a graphable number to each of your dyed test swatches of fabric.
Good luck with your project.
(Please help support this web site. Thank you.)
Friday, November 17, 2006
safe non-toxic dyes for dog toys
Name:
Helen
Message: I am making dog toys from hemp rope/canvas and also loofah which I would like to dye. I am concerned that the toys will be chewed and potentially eaten so want to find a safe non toxic dye that is colour fast and also eco friendly. I was going to use natural vegetable dyes but the dyeing process seems very complicated and not necessarily safe! Please could you recommend a suitable dye that would be non toxic and as eco friendly as possible?
You can't use the safest class of dyes, which are the food colorings, because they will not work on cellulose fibers such as hemp or loofah. They will work only on nylon or on animal fibers, such as wool.
As you have seen, most natural dyes require mordants, which would not be suitable for dogs to chew on. The least toxic mordant is alum, but even that can be irritating and slightly toxic. (The fatal dose for an adult human is about one ounce.)
One natural dye that works well on plant fibers is turmeric. Unlike the majority of natural dyes, turmeric is a direct dye, which means that it will dye cellulose fibers with no mordant at all. The color produced is a bright orangish yellow. Turmeric is not a very good dye for clothing, because it tends to fade in the light, and in the laundry as well. It seems likely to me that neither of these will be a huge drawback for dog toys, which tend to have a fairly short lifespan. Turmeric is certainly worth your running some tests. Try simmering one dog toy for half an hour in a saucepan of water which covers the toy completely, with one to four tablespoons of ground dried turmeric mixed into the water. Adding a few spoonfuls of salt might be helpful. Look in the grocery store for ground turmeric; you'll probably find the prices dramatically cheaper if you can buy it in bulk rather than in tiny glass jars.
Walnut hulls will also produce a good direct dye for cellulose, but the dark brown color may not be much fun for dog toys. You must gather pounds of walnut hulls if you are going to dye with them.
Another good choice for dyeing cellulose fibers such as hemp and loofah would be fiber reactive dyes. These are considered to be more ecologically sound for use than other classes of synthetic dyes. As a general rule, fiber reactive dyes are not appreciably toxic if they have been fixed to the fiber properly, with soda ash, and all excess unattached dye has been washed out, using very hot water (140°F) after an initial cool rinse. However, none of the fiber reactive dyes have been tested for safety when eaten. The most popular fiber reactive dye is Procion MX dye. This is the same dye that is so popular for use in tie-dyeing clothing. The dye actually forms a permanent chemical bond with the fiber, which cannot be broken even by boiling. There is always quite a bit of unattached dye which must be washed out, but once you have done that, what's left is quite permanent. Dylon Machine Dye is a fiber reactive dye which would be worth your trying, if you can find it. For recipes, see How to Dye with Fiber Reactive Dye and Washing Machine Dyeing. To find a local source from which to mail-order fiber reactive dyes, see my page of Sources for Dyeing Supplies Around the World .
(Please help support this web site. Thank you.)
[Updated January 14, 2009.]
Message: I am making dog toys from hemp rope/canvas and also loofah which I would like to dye. I am concerned that the toys will be chewed and potentially eaten so want to find a safe non toxic dye that is colour fast and also eco friendly. I was going to use natural vegetable dyes but the dyeing process seems very complicated and not necessarily safe! Please could you recommend a suitable dye that would be non toxic and as eco friendly as possible?
You can't use the safest class of dyes, which are the food colorings, because they will not work on cellulose fibers such as hemp or loofah. They will work only on nylon or on animal fibers, such as wool.
As you have seen, most natural dyes require mordants, which would not be suitable for dogs to chew on. The least toxic mordant is alum, but even that can be irritating and slightly toxic. (The fatal dose for an adult human is about one ounce.)
One natural dye that works well on plant fibers is turmeric. Unlike the majority of natural dyes, turmeric is a direct dye, which means that it will dye cellulose fibers with no mordant at all. The color produced is a bright orangish yellow. Turmeric is not a very good dye for clothing, because it tends to fade in the light, and in the laundry as well. It seems likely to me that neither of these will be a huge drawback for dog toys, which tend to have a fairly short lifespan. Turmeric is certainly worth your running some tests. Try simmering one dog toy for half an hour in a saucepan of water which covers the toy completely, with one to four tablespoons of ground dried turmeric mixed into the water. Adding a few spoonfuls of salt might be helpful. Look in the grocery store for ground turmeric; you'll probably find the prices dramatically cheaper if you can buy it in bulk rather than in tiny glass jars.
Walnut hulls will also produce a good direct dye for cellulose, but the dark brown color may not be much fun for dog toys. You must gather pounds of walnut hulls if you are going to dye with them.
Another good choice for dyeing cellulose fibers such as hemp and loofah would be fiber reactive dyes. These are considered to be more ecologically sound for use than other classes of synthetic dyes. As a general rule, fiber reactive dyes are not appreciably toxic if they have been fixed to the fiber properly, with soda ash, and all excess unattached dye has been washed out, using very hot water (140°F) after an initial cool rinse. However, none of the fiber reactive dyes have been tested for safety when eaten. The most popular fiber reactive dye is Procion MX dye. This is the same dye that is so popular for use in tie-dyeing clothing. The dye actually forms a permanent chemical bond with the fiber, which cannot be broken even by boiling. There is always quite a bit of unattached dye which must be washed out, but once you have done that, what's left is quite permanent. Dylon Machine Dye is a fiber reactive dye which would be worth your trying, if you can find it. For recipes, see How to Dye with Fiber Reactive Dye and Washing Machine Dyeing. To find a local source from which to mail-order fiber reactive dyes, see my page of Sources for Dyeing Supplies Around the World .
(Please help support this web site. Thank you.)
[Updated January 14, 2009.]
Thursday, November 16, 2006
Could we successfully dye the veil to match, by Saturday?
Name: Janet
Message: My daughter was given a bridal veil (netting with appliques) that is white. Her dress is ivory. Could we successfully dye the veil to match? If yes, how? We only have 5 days to the wedding!
Dyeing an item of unknown fiber content, just days before it is needed, is a very risky procedure. What will you do if the results are not suitable? Normally, it takes quite a lot of experience to be able to duplicate a desired color. The idea of novices doing this as their very first dyeing project fills me with dread.
The veil itself is probably made of nylon. Nylon picks up dye very readily in the presence of vinegar or other mild acid, when heated in a mixture of dye and water. If your veil contained only nylon, without appliqués, this project might be a reasonable one. You could simmer your veil in coffee for a while, until it picked up the desired tone, then rinse it out thoroughly. However, different fibers take dye to drastically different levels. If the veil is polyester, it will take up a little coffee color after it has boiled for a good long while; if the appliqués are made of nylon, they will take the color far more strongly.
Depending on the actual fiber content of the veil and the appliqués, you might end up with a tan veil with tan appliqués—or you might end up with a brown veil, or a white veil with tan appliqués, or a tan veil with white appliqués.
I am sorry that I cannot be more encouraging about this last-minute project. It's just impossible to predict what will happen if you attempt to dye this veil, given the fact that the fiber contents of the different parts of the veil are unknown. Disaster is not at all unlikely. It is best to embark on such unpredictable projects at least several months in advance of their projected use; it's unfortunate that you were given this veil so much at the last minute. It would be wiser to use the veil in its current color than to attempt to dye it by this weekend.
(Please help support this web site. Thank you.)
Message: My daughter was given a bridal veil (netting with appliques) that is white. Her dress is ivory. Could we successfully dye the veil to match? If yes, how? We only have 5 days to the wedding!
Dyeing an item of unknown fiber content, just days before it is needed, is a very risky procedure. What will you do if the results are not suitable? Normally, it takes quite a lot of experience to be able to duplicate a desired color. The idea of novices doing this as their very first dyeing project fills me with dread.
The veil itself is probably made of nylon. Nylon picks up dye very readily in the presence of vinegar or other mild acid, when heated in a mixture of dye and water. If your veil contained only nylon, without appliqués, this project might be a reasonable one. You could simmer your veil in coffee for a while, until it picked up the desired tone, then rinse it out thoroughly. However, different fibers take dye to drastically different levels. If the veil is polyester, it will take up a little coffee color after it has boiled for a good long while; if the appliqués are made of nylon, they will take the color far more strongly.
Depending on the actual fiber content of the veil and the appliqués, you might end up with a tan veil with tan appliqués—or you might end up with a brown veil, or a white veil with tan appliqués, or a tan veil with white appliqués.
I am sorry that I cannot be more encouraging about this last-minute project. It's just impossible to predict what will happen if you attempt to dye this veil, given the fact that the fiber contents of the different parts of the veil are unknown. Disaster is not at all unlikely. It is best to embark on such unpredictable projects at least several months in advance of their projected use; it's unfortunate that you were given this veil so much at the last minute. It would be wiser to use the veil in its current color than to attempt to dye it by this weekend.
(Please help support this web site. Thank you.)
Wednesday, November 15, 2006
While I see how both cotton and polyester are to be died, what do you do in the case of an article being 65% polyester and 35% cotton?
Name: Daniel
Message: While I see how both cotton and polyester are to be dyed, what do you do in the case of an article being 65% polyester and 35% cotton?
You have two choices: either you choose to dye just one of the two fibers, leaving the other undyed, or you dye the two fibers with two different dyes.
There is no cotton dye that will permanently color polyester, and there is no polyester dye that will permanently dye cotton.
If you use a cotton dye, such as fiber reactive dye or direct dye, you will get a pale or muted shade, since only 35% of the fiber will accept the dye. If you dye only the polyester, using disperse dye and the appropriate carrier chemical, you will get a more acceptable final color; omitting the carrier chemical will result in a paler shade.
If you choose a high quality fiber reactive dye, it must be applied in a separate dyebath from the polyester dye. Fiber reactive dye uses much lower reaction temperatures and different chemical auxiliaries.
If you would prefer to use both dyes in a single dyebath, dyeing the polyester boiling your garment for an hour with disperse dye and the appropriate carrier chemical, you will probably be able to also apply direct dye in the same step. However, this does not really save trouble, since direct dye requires an addition dye fixative treatment, after dyeing has been completed. Direct dye is not acceptably washfast, so you must apply a cationic dye fixative after dyeing has been completed.
(Please help support this web site. Thank you.)
Message: While I see how both cotton and polyester are to be dyed, what do you do in the case of an article being 65% polyester and 35% cotton?
You have two choices: either you choose to dye just one of the two fibers, leaving the other undyed, or you dye the two fibers with two different dyes.
There is no cotton dye that will permanently color polyester, and there is no polyester dye that will permanently dye cotton.
If you use a cotton dye, such as fiber reactive dye or direct dye, you will get a pale or muted shade, since only 35% of the fiber will accept the dye. If you dye only the polyester, using disperse dye and the appropriate carrier chemical, you will get a more acceptable final color; omitting the carrier chemical will result in a paler shade.
If you choose a high quality fiber reactive dye, it must be applied in a separate dyebath from the polyester dye. Fiber reactive dye uses much lower reaction temperatures and different chemical auxiliaries.
If you would prefer to use both dyes in a single dyebath, dyeing the polyester boiling your garment for an hour with disperse dye and the appropriate carrier chemical, you will probably be able to also apply direct dye in the same step. However, this does not really save trouble, since direct dye requires an addition dye fixative treatment, after dyeing has been completed. Direct dye is not acceptably washfast, so you must apply a cationic dye fixative after dyeing has been completed.
(Please help support this web site. Thank you.)
Tuesday, November 14, 2006
mixing a true red with Procion MX dyes
Name: Jami
Message: You recently mentioned in the Dyer's list mailing list: "Orange MX-2R is useful for mixing a true red" (Nov 4) Could you tell me how to do this? You don't list true red in your list of FAQ to get specific colors.
Try mixing equal parts of orange MX-2R with red MX-5B. (See my list of "Which Procion MX colors are pure, and which mixtures?" to translate these codes to the catalog names and numbers of these dyes at various suppliers.) I believe that these are the dyes in the mixture used in Jacquard Fire Engine Red, and it's a good one. You may need to adjust the proportions to taste, and, as with any fiber reactive dye, the proportions needed will be different for different fibers. If you measure by weight, your proportions will be more reproducible, for one type of fiber, than if you measure by teaspoons, but measuring by teaspoons is less trouble, if you are as not much concerned with reproducibility.
You can also get a true red by mixing a small amount of any yellow, including yellow MX-8G, yellow MX-GR, or yellow MX-3R(A), with either red MX-5B or red MX-8B. I like the mixture of red MX-5B with orange MX-2R because it does not make yellow halos, as the popular mixture of yellow MX-8G with red MX-8B does, and red MX-5B tends to dissolve better than red MX-8B.
There is a true red among the dichlorotriazine (Procion MX type) dyes, red MX-G, or reactive red 5, but I did not find it to be superior in any way to the mixture of orange MX-2R with red MX-5B, and it was much more expensive. Red MX-G is also very hard to find; none of the major dye retailers carry it.
(Please help support this web site. Thank you.)
Message: You recently mentioned in the Dyer's list mailing list: "Orange MX-2R is useful for mixing a true red" (Nov 4) Could you tell me how to do this? You don't list true red in your list of FAQ to get specific colors.
Try mixing equal parts of orange MX-2R with red MX-5B. (See my list of "Which Procion MX colors are pure, and which mixtures?" to translate these codes to the catalog names and numbers of these dyes at various suppliers.) I believe that these are the dyes in the mixture used in Jacquard Fire Engine Red, and it's a good one. You may need to adjust the proportions to taste, and, as with any fiber reactive dye, the proportions needed will be different for different fibers. If you measure by weight, your proportions will be more reproducible, for one type of fiber, than if you measure by teaspoons, but measuring by teaspoons is less trouble, if you are as not much concerned with reproducibility.
You can also get a true red by mixing a small amount of any yellow, including yellow MX-8G, yellow MX-GR, or yellow MX-3R(A), with either red MX-5B or red MX-8B. I like the mixture of red MX-5B with orange MX-2R because it does not make yellow halos, as the popular mixture of yellow MX-8G with red MX-8B does, and red MX-5B tends to dissolve better than red MX-8B.
There is a true red among the dichlorotriazine (Procion MX type) dyes, red MX-G, or reactive red 5, but I did not find it to be superior in any way to the mixture of orange MX-2R with red MX-5B, and it was much more expensive. Red MX-G is also very hard to find; none of the major dye retailers carry it.
(Please help support this web site. Thank you.)
Monday, November 13, 2006
problems with direct application of indigo dye
Name: paulette george
Message: I am a graduate student, I have searched hi and lo for information on if it is possible to directly apply Indigo dye. I would like to use it in silk screening if it is possible. I have tried sodium alge. as a thickener without luck. Please help!
Indigo dye should be applied in a reduced form, which is called the "leuko" form, because, like all vat dyes, it is only when it is reduced that indigo is soluble and able to get inside a fiber. Otherwise it will just sit on the surface and will not be permanent in the fabric.
I'm not sure how much chemistry you know. "Reduced" is the opposite of "oxidized", in chemistry; a vat dye such as indigo can be converted from oxidized to reduced form, and back again, depending on the chemicals and amount of air around it. Air oxidizes indigo to its insoluble form. The reduced form of blue indigo is yellow in color. The oxidized form of indigo is blue.
Using a dye thickener such as sodium alginate will not help. The issue is the chemistry of vat dyes.
If indigo is applied when it is in the fully oxidized blue form, it will not be able to get inside the fiber and will rub off (in a process called crocking) when dry, and will also wash out.
To reduce indigo, you can prepare a natural fermentation dyebath with bran, or a thiourea dioxide/lye dyebath, or a zinc/lime dye bath. Each of these presents its own challenges. Sometimes you can also buy pre-reduced freeze-dried indigo crystals from Aurora Silk, and it looks as though Paradise Fibers may have it in stock currently. Directly painted indigo, even in reduced form, could end up much less permanently attached than indigo that has been properly applied in a dye vat, depending on how much it gets oxidized before it actually penetrates the fiber. It is best to immerse fabric in an indigo dyebath, as any surface application of indigo is bound to expose it to oxygen, which will convert it to the insoluble form. However, if you are determined to do direct application of indigo dye, there is no question but that pre-reduced indigo is the only way to go. There's just no point at all in attempting painting or silk=screening with oxidized indigo.
Indigotine, also called indigo carmine, is a chemical derivative of indigo which has been treated to make it soluble in water, by adding sulfate groups. It is not used by natural dyers, but it is commercially available as a food dye, US FD&C Blue No. 2, and is labeled E132 in Europe. Unfortunately, the blue food dye you normally find available is US FD&C blue 1, a completely different dye. Like other food dyes, Indigotine cannot be used to dye cotton, because it is an acid dye and cannot attach to cotton, but it can probably be used quite well to dye wool or silk; it will be necessary to steam wool or silk that has been painted with indigotine, in order to fix the mostly hydrogen bonds that hold the acid dye to the fabric. Steaming will not help to fix true indigo to silk or wool, but it will probably work nicely for indigotine.
A more suitable type of dye for silk-screening would be fiber reactive dye, such as Procion MX. This dye, thickened with alginate or another dye thickener, is applied to fabric that has been pre-treated with soda ash. If properly applied, it is extremely washfast and long-lasting. Fabric paint is more commonly used for silk screening, but it is certainly very possible to use the right kind of dye, instead.
(Please help support this web site. Thank you.)
Message: I am a graduate student, I have searched hi and lo for information on if it is possible to directly apply Indigo dye. I would like to use it in silk screening if it is possible. I have tried sodium alge. as a thickener without luck. Please help!
Indigo dye should be applied in a reduced form, which is called the "leuko" form, because, like all vat dyes, it is only when it is reduced that indigo is soluble and able to get inside a fiber. Otherwise it will just sit on the surface and will not be permanent in the fabric.
I'm not sure how much chemistry you know. "Reduced" is the opposite of "oxidized", in chemistry; a vat dye such as indigo can be converted from oxidized to reduced form, and back again, depending on the chemicals and amount of air around it. Air oxidizes indigo to its insoluble form. The reduced form of blue indigo is yellow in color. The oxidized form of indigo is blue.
Using a dye thickener such as sodium alginate will not help. The issue is the chemistry of vat dyes.
If indigo is applied when it is in the fully oxidized blue form, it will not be able to get inside the fiber and will rub off (in a process called crocking) when dry, and will also wash out.
To reduce indigo, you can prepare a natural fermentation dyebath with bran, or a thiourea dioxide/lye dyebath, or a zinc/lime dye bath. Each of these presents its own challenges. Sometimes you can also buy pre-reduced freeze-dried indigo crystals from Aurora Silk, and it looks as though Paradise Fibers may have it in stock currently. Directly painted indigo, even in reduced form, could end up much less permanently attached than indigo that has been properly applied in a dye vat, depending on how much it gets oxidized before it actually penetrates the fiber. It is best to immerse fabric in an indigo dyebath, as any surface application of indigo is bound to expose it to oxygen, which will convert it to the insoluble form. However, if you are determined to do direct application of indigo dye, there is no question but that pre-reduced indigo is the only way to go. There's just no point at all in attempting painting or silk=screening with oxidized indigo.
Indigotine, also called indigo carmine, is a chemical derivative of indigo which has been treated to make it soluble in water, by adding sulfate groups. It is not used by natural dyers, but it is commercially available as a food dye, US FD&C Blue No. 2, and is labeled E132 in Europe. Unfortunately, the blue food dye you normally find available is US FD&C blue 1, a completely different dye. Like other food dyes, Indigotine cannot be used to dye cotton, because it is an acid dye and cannot attach to cotton, but it can probably be used quite well to dye wool or silk; it will be necessary to steam wool or silk that has been painted with indigotine, in order to fix the mostly hydrogen bonds that hold the acid dye to the fabric. Steaming will not help to fix true indigo to silk or wool, but it will probably work nicely for indigotine.
A more suitable type of dye for silk-screening would be fiber reactive dye, such as Procion MX. This dye, thickened with alginate or another dye thickener, is applied to fabric that has been pre-treated with soda ash. If properly applied, it is extremely washfast and long-lasting. Fabric paint is more commonly used for silk screening, but it is certainly very possible to use the right kind of dye, instead.
(Please help support this web site. Thank you.)
Sunday, November 12, 2006
I am thinking of purchasing a white 100% rayon velvet wedding gown w/the intentions of dyeing it blue. I've never dyed anything in my life. First of all is this possible?
Name: APRIL
Message: Hello. I am thinking of purchasing a white 100% rayon velvet wedding gown w/the intentions of dyeing it blue. I've never dyed anything in my life. First of all is this possible? If so can you let me know what I need to do/buy? Thanks so much!
100% rayon, if it is viscose rayon and not undyeable rayon acetate, dyes brightly and well with a cold water fiber reactive dye such as Procion MX. Rayon velvet usually dyes wonderfully. Do not use a hot water dye, such as all-purpose dye, on a rayon velvet dress.
However, the dress must be washable in order to be dyeable, and dyeing your own wedding dress is a bit more complicated and high-risk a project than I would recommend for a beginner. Try dyeing some t-shirts first! You will need to buy some Procion MX dyes by mail-order from one of the retailers listed on my Sources for Dyeing Supplies page, soda ash, and a large amount of salt.
The easiest way to dye a washable rayon dress to a solid color is in the washing machine. If your washing machine has a hand-wash/delicate setting, you may be able to dye a hand-wash-only dress, if you are careful. Do not try to dye anything that is marked as dry-clean-only, especially if it is lined. Linings are usually made of undeclared synthetics, and when the outside layer of a garment shrinks more than the lining, the garment is usually ruined.
Make sure that the dress you buy was sewn with cotton thread. Polyester thread, which is used to sew together almost all clothing, will remain white after dyeing. Also, allow plenty of time for this project, so that if you decide that you do not like the results, you will still have time to go buy another dress.
(Please help support this web site. Thank you.)
Message: Hello. I am thinking of purchasing a white 100% rayon velvet wedding gown w/the intentions of dyeing it blue. I've never dyed anything in my life. First of all is this possible? If so can you let me know what I need to do/buy? Thanks so much!
100% rayon, if it is viscose rayon and not undyeable rayon acetate, dyes brightly and well with a cold water fiber reactive dye such as Procion MX. Rayon velvet usually dyes wonderfully. Do not use a hot water dye, such as all-purpose dye, on a rayon velvet dress.
However, the dress must be washable in order to be dyeable, and dyeing your own wedding dress is a bit more complicated and high-risk a project than I would recommend for a beginner. Try dyeing some t-shirts first! You will need to buy some Procion MX dyes by mail-order from one of the retailers listed on my Sources for Dyeing Supplies page, soda ash, and a large amount of salt.
The easiest way to dye a washable rayon dress to a solid color is in the washing machine. If your washing machine has a hand-wash/delicate setting, you may be able to dye a hand-wash-only dress, if you are careful. Do not try to dye anything that is marked as dry-clean-only, especially if it is lined. Linings are usually made of undeclared synthetics, and when the outside layer of a garment shrinks more than the lining, the garment is usually ruined.
Make sure that the dress you buy was sewn with cotton thread. Polyester thread, which is used to sew together almost all clothing, will remain white after dyeing. Also, allow plenty of time for this project, so that if you decide that you do not like the results, you will still have time to go buy another dress.
(Please help support this web site. Thank you.)
Saturday, November 11, 2006
My batiked shirts never seem as vibrant as just tie-dyed shirts. Any suggestions?
Name: Chris L.
Message: Hi there, I've been experimenting with batik on cotton t-shirts, and I always seem to have trouble that the dye fades during the boiling out of wax. I've tried rinsing in cold water, then washing in cold with synthrapol first, then boiling out, as well as boiling out first, then washing in synthrapol like you would without wax. But my shirts never seem as vibrant as just tie-dyed shirts. Any suggestions? I love your website, by the way! Thanks a lot.
What kind of dye are you using? What brand name is it, and where are you buying it? How are you setting the dye?
Batik should be just as brilliant and vibrant in color as the brightest tie-dyes, if you use the right dye and do everything right in applying it. Applying the right dye is easy, but sometimes there is some detail that someone does not get right. If we can figure out that that is, you'll be able to get colors just as bright as you want.
I've been using the Procion dye from Dharma Trading. I've followed the instructions they send with their tie dye kit, which involves water, urea, salt and the dye. After washing and drying new shirts, I wax my design, then soak in soda ash for 15-20 minutes, before dying. Sometimes I dye by squirting on with bottles, then rubbing it in a bit to blend the colours, other times I paint it on with a paint brush. Not too much in the vat dye method, but maybe that would be a better choice? I think the recipe for the dye is different then.
That's a good choice of dye, assuming that your dyes are fresh (no more than a year old) and have never been stored in a very hot place (such as a few hours in a hot car). You should not need to change to vat-dyeing instead of direct dye application. There are several things you might be doing wrong:
1. You might not be using enough dye. I recommend four teaspoons of dye per cup of dye solution. Your dye on the shirt should look way too dark before you wash it out.
2. You might have hard water. If so, always use water softener, sodium hexametaphosphate, often sold by dye suppliers as Metaphos. Hard water dulls dye colors and also makes it hard to wash out excess unattached dye afterwards. Add it to your dye solutions, your soda ash, and your washing and rinsing cycles in the washing machine.
3. You might not be giving the reaction between the dye and the fabric (with the soda ash!) enough warmth and/or time. The minimum temperature is 70°F overnight (21°C). I prefer to find a warmer place, at least 80°F, such as the top of the refrigerator, the top of the water heater, in a bucket in a sinkful of hot water, or covered with a plastic sheet and an electric blanket.
4. Your colors may be being dulled by backstaining. Before washing out, allow excess time for all of the dye to react, so there is no unreacted dye to stain other parts of the design permanently, and do your final dye washout with HOT (140°F) water, to get out all excess unattached dye.
5. Shirts with any stain-resistant or permanent-press finish, or with any synthetic fiber content other than rayon, will not dye brightly. Use only 100% cotton with no surface finishes.
6. You might be exposing your dyes to soda ash too soon. Dye will go bad within an hour or so after it is first exposed to soda ash. This includes dye that has had the paint brush put directly into it after touching the soda-soaked shirt. The paint brush gets soda ash from the shirt into the dye you are painting with, even if you dried your soda-soaked shirt before starting to paint on it. Use a small temporary container of dye for directly painting your pre-soaked shirts, and toss it out and get more dye from your main dye mixture after an hour of use.
7. This may sound ridiculous, but I've known cases in which there was no soda ash in the soda ash soak! A different white powder, most likely urea, had been inadvertently used to mix up what everyone thought was the soda ash soak. Check your labels carefully. Unknowingly omitting soda ash will really dull your colors down.
8. It sounds like you're doing a single round of waxing and dyeing, but if you're doing the traditional multiple rounds of waxing and dyeing, color choice is a real key in the brightness you get. Any time that a given section of fabric gets all three primary colors on it (turquoise or blue, magenta or red, and yellow), it will end up being duller in color. This can be a great thing when the darker, duller colors are what you want, but it's something to be keenly aware of.
9. Color choice in general makes a big difference in brightness. For the very brightest mixed colors, use yellow MX-G for your yellow, turquoise MX-G for your blue, and either red MX-5B or red MX-8B for your red. (Red MX-5B is easier to dissolve and will not leave the dots of red on your work that red MX-8B and mixtures containing it often will.)
Also, when I mix a couple of colours together (ie turquoise and yellow to make green), do I need to wait a while to let them combine? Sometimes they don't seem to blend well, or when I rinse out the yellow seems to rinse out more than the turquoise. I always mix them together after they are in the liquid form, not the powder form. I had a similar experience mixing green (which was made up of the turquoise/yellow) and red to get brown. In the rinsing process, more green rinsed out leaving a more reddish brown.
When you mix different colors, they do not react together in any way. If one color rinses out more than another, it is because it did not react well on the fabric. When turquoise rinses out more than other colors, it usually means your room temperature is too low, because turquoise needs extra warmth compared to the other Procion MX dye colors. If yellow is rinsing out too much and leaving you with less yellow on your piece, you either need to use more yellow, or you need to buy a fresh jar. Any of the problems listed above may affect one dye color more than another.
Properly affixed Procion MX dye will survive any washing you care to give it, including boiling. I like to rinse once in cool water, and then wash a couple of times in hot (140°F) water. The only Procion MX dye that is removed when you boil out the wax is dye that was never permanently bonded to the fabric in the first place. There is always a lot of excess unattached dye to wash out, so if you have not completed the washout, you will see some dye in your wax boiling water, but this should not affect the brightness of your fabric if you do everything right to get a good amount of dye fixation to the fabric during the warm dye/fiber reaction time (with soda ash).
(Please help support this web site. Thank you.)
Message: Hi there, I've been experimenting with batik on cotton t-shirts, and I always seem to have trouble that the dye fades during the boiling out of wax. I've tried rinsing in cold water, then washing in cold with synthrapol first, then boiling out, as well as boiling out first, then washing in synthrapol like you would without wax. But my shirts never seem as vibrant as just tie-dyed shirts. Any suggestions? I love your website, by the way! Thanks a lot.
What kind of dye are you using? What brand name is it, and where are you buying it? How are you setting the dye?
Batik should be just as brilliant and vibrant in color as the brightest tie-dyes, if you use the right dye and do everything right in applying it. Applying the right dye is easy, but sometimes there is some detail that someone does not get right. If we can figure out that that is, you'll be able to get colors just as bright as you want.
I've been using the Procion dye from Dharma Trading. I've followed the instructions they send with their tie dye kit, which involves water, urea, salt and the dye. After washing and drying new shirts, I wax my design, then soak in soda ash for 15-20 minutes, before dying. Sometimes I dye by squirting on with bottles, then rubbing it in a bit to blend the colours, other times I paint it on with a paint brush. Not too much in the vat dye method, but maybe that would be a better choice? I think the recipe for the dye is different then.
That's a good choice of dye, assuming that your dyes are fresh (no more than a year old) and have never been stored in a very hot place (such as a few hours in a hot car). You should not need to change to vat-dyeing instead of direct dye application. There are several things you might be doing wrong:
1. You might not be using enough dye. I recommend four teaspoons of dye per cup of dye solution. Your dye on the shirt should look way too dark before you wash it out.
2. You might have hard water. If so, always use water softener, sodium hexametaphosphate, often sold by dye suppliers as Metaphos. Hard water dulls dye colors and also makes it hard to wash out excess unattached dye afterwards. Add it to your dye solutions, your soda ash, and your washing and rinsing cycles in the washing machine.
3. You might not be giving the reaction between the dye and the fabric (with the soda ash!) enough warmth and/or time. The minimum temperature is 70°F overnight (21°C). I prefer to find a warmer place, at least 80°F, such as the top of the refrigerator, the top of the water heater, in a bucket in a sinkful of hot water, or covered with a plastic sheet and an electric blanket.
4. Your colors may be being dulled by backstaining. Before washing out, allow excess time for all of the dye to react, so there is no unreacted dye to stain other parts of the design permanently, and do your final dye washout with HOT (140°F) water, to get out all excess unattached dye.
5. Shirts with any stain-resistant or permanent-press finish, or with any synthetic fiber content other than rayon, will not dye brightly. Use only 100% cotton with no surface finishes.
6. You might be exposing your dyes to soda ash too soon. Dye will go bad within an hour or so after it is first exposed to soda ash. This includes dye that has had the paint brush put directly into it after touching the soda-soaked shirt. The paint brush gets soda ash from the shirt into the dye you are painting with, even if you dried your soda-soaked shirt before starting to paint on it. Use a small temporary container of dye for directly painting your pre-soaked shirts, and toss it out and get more dye from your main dye mixture after an hour of use.
7. This may sound ridiculous, but I've known cases in which there was no soda ash in the soda ash soak! A different white powder, most likely urea, had been inadvertently used to mix up what everyone thought was the soda ash soak. Check your labels carefully. Unknowingly omitting soda ash will really dull your colors down.
8. It sounds like you're doing a single round of waxing and dyeing, but if you're doing the traditional multiple rounds of waxing and dyeing, color choice is a real key in the brightness you get. Any time that a given section of fabric gets all three primary colors on it (turquoise or blue, magenta or red, and yellow), it will end up being duller in color. This can be a great thing when the darker, duller colors are what you want, but it's something to be keenly aware of.
9. Color choice in general makes a big difference in brightness. For the very brightest mixed colors, use yellow MX-G for your yellow, turquoise MX-G for your blue, and either red MX-5B or red MX-8B for your red. (Red MX-5B is easier to dissolve and will not leave the dots of red on your work that red MX-8B and mixtures containing it often will.)
Also, when I mix a couple of colours together (ie turquoise and yellow to make green), do I need to wait a while to let them combine? Sometimes they don't seem to blend well, or when I rinse out the yellow seems to rinse out more than the turquoise. I always mix them together after they are in the liquid form, not the powder form. I had a similar experience mixing green (which was made up of the turquoise/yellow) and red to get brown. In the rinsing process, more green rinsed out leaving a more reddish brown.
When you mix different colors, they do not react together in any way. If one color rinses out more than another, it is because it did not react well on the fabric. When turquoise rinses out more than other colors, it usually means your room temperature is too low, because turquoise needs extra warmth compared to the other Procion MX dye colors. If yellow is rinsing out too much and leaving you with less yellow on your piece, you either need to use more yellow, or you need to buy a fresh jar. Any of the problems listed above may affect one dye color more than another.
Properly affixed Procion MX dye will survive any washing you care to give it, including boiling. I like to rinse once in cool water, and then wash a couple of times in hot (140°F) water. The only Procion MX dye that is removed when you boil out the wax is dye that was never permanently bonded to the fabric in the first place. There is always a lot of excess unattached dye to wash out, so if you have not completed the washout, you will see some dye in your wax boiling water, but this should not affect the brightness of your fabric if you do everything right to get a good amount of dye fixation to the fabric during the warm dye/fiber reaction time (with soda ash).
(Please help support this web site. Thank you.)
Friday, November 10, 2006
Do I really need to use fixative with Dylon Cold Dye, or is that them trying to get me to buy more?
Name: Claudia
Message: Hi, I have purchased some Dylon COLD A52 black dye to dye a white cotton t shirt, I have read the instructions but do i really need a fixing thing or is that them trying to get me to buy more? Thankyou, this website is great!
You really do need to use a fixative with your Dylon Cold dye. You can buy their fixative, or you can get some soda ash (sodium carbonate) from a swimming pool supply store. Be careful to read the fine print; do not buy sodium BIcarbonate, which is baking soda and will not work. Alternatively, you can use three times the quantity called for of washing soda. Washing soda contains the same chemical as soda ash, but is only one-third as strong.
Be sure to place your shirt + dye + fixative in a warm place to react, preferably 80°F or higher, overnight. At least some of the dyes in Dylon Cold Water Dye #52 Black are Drimarene K type dyes. These are a high quality fiber reactive dye, but require a little more warmth to react than Procion MX type dye. The optimum reaction temperature for Drimarene K dyes is 40 to 60°C, depending on the dye color, which translates to 104°F to 140°F.
You can see the Dylon Dye company's instructions for their cold water dyes at http://www.dylon.co.uk/colourcentre/coldwdye/coldwdye.htm . Their instructions call for one sachet of Cold Fix; substitute either 15 grams of soda ash, which is about 25 ml (five teaspoons), or 75 ml (five tablespoons) of washing soda.
(Please help support this web site. Thank you.)
Message: Hi, I have purchased some Dylon COLD A52 black dye to dye a white cotton t shirt, I have read the instructions but do i really need a fixing thing or is that them trying to get me to buy more? Thankyou, this website is great!
You really do need to use a fixative with your Dylon Cold dye. You can buy their fixative, or you can get some soda ash (sodium carbonate) from a swimming pool supply store. Be careful to read the fine print; do not buy sodium BIcarbonate, which is baking soda and will not work. Alternatively, you can use three times the quantity called for of washing soda. Washing soda contains the same chemical as soda ash, but is only one-third as strong.
Be sure to place your shirt + dye + fixative in a warm place to react, preferably 80°F or higher, overnight. At least some of the dyes in Dylon Cold Water Dye #52 Black are Drimarene K type dyes. These are a high quality fiber reactive dye, but require a little more warmth to react than Procion MX type dye. The optimum reaction temperature for Drimarene K dyes is 40 to 60°C, depending on the dye color, which translates to 104°F to 140°F.
You can see the Dylon Dye company's instructions for their cold water dyes at http://www.dylon.co.uk/colourcentre/coldwdye/coldwdye.htm . Their instructions call for one sachet of Cold Fix; substitute either 15 grams of soda ash, which is about 25 ml (five teaspoons), or 75 ml (five tablespoons) of washing soda.
(Please help support this web site. Thank you.)
Saturday, November 04, 2006
How can I get my dyed towels and washcloths to quit bleeding out? I've washed and rinsed and soaked these articles well over 50 times and they still 'bleed'.
Name: Jean
Message: How can I get my dyed towels and washcloths to quit bleeding out? I've washed and rinsed and soaked these articles well over 50 times and they still 'bleed'. HELP PLEASE!!! THANK YOU VERY MUCH FOR YOUR TIME.
Never buy towels from that manufacturer again. Dye that bleeds forever is an unacceptable manufacturing defect. They should have been returned to the seller for a refund.
Neither salt nor vinegar, two old home remedies often suggested by people who don't know what they're talking about, will help your situation. Fortunately, there is another product available now which will work quite well for your problem.
What you can do now is buy some of a special commercial dye fixative known as Retayne. Unless you have a well-equipped quilter's supply store nearby, you will have to order this product by mail. You can buy this product from most of the dye suppliers listed on my Sources for Dyeing Supplies Around the World page. There are also other brands of dye fixative that will accomplish the same thing such as Raycafix sold by G&S Dye, Dharma Dye Fixative sold by Dharma Trading Company, and so on. The generic classification of these products is "cationic dye fixatives".
Follow the instructions supplied with the bottle. Usually you just have to add a small amount of the dye fixative while running a washing machine load of fabric. In the future, wash the items in cool water to prolong the effects of the dye fixative, which may wash out in hot water. If you must wash in hot water to remove stains, don't worry, you can always apply the Retayne again.
For more information, see "Commercial Dye Fixatives".
(Please help support this web site. Thank you.)
Message: How can I get my dyed towels and washcloths to quit bleeding out? I've washed and rinsed and soaked these articles well over 50 times and they still 'bleed'. HELP PLEASE!!! THANK YOU VERY MUCH FOR YOUR TIME.
Never buy towels from that manufacturer again. Dye that bleeds forever is an unacceptable manufacturing defect. They should have been returned to the seller for a refund.
Neither salt nor vinegar, two old home remedies often suggested by people who don't know what they're talking about, will help your situation. Fortunately, there is another product available now which will work quite well for your problem.
What you can do now is buy some of a special commercial dye fixative known as Retayne. Unless you have a well-equipped quilter's supply store nearby, you will have to order this product by mail. You can buy this product from most of the dye suppliers listed on my Sources for Dyeing Supplies Around the World page. There are also other brands of dye fixative that will accomplish the same thing such as Raycafix sold by G&S Dye, Dharma Dye Fixative sold by Dharma Trading Company, and so on. The generic classification of these products is "cationic dye fixatives".
Follow the instructions supplied with the bottle. Usually you just have to add a small amount of the dye fixative while running a washing machine load of fabric. In the future, wash the items in cool water to prolong the effects of the dye fixative, which may wash out in hot water. If you must wash in hot water to remove stains, don't worry, you can always apply the Retayne again.
For more information, see "Commercial Dye Fixatives".
(Please help support this web site. Thank you.)
Wednesday, November 01, 2006
beautiful raindrop and malachite effects from salt and alcohol in silk painting
Name:
Cathy 
Message: I have read and combed over your website and had great fun with it, especially the low water immersion dyeing information. A zillion thank you's for your kind work.
I saw some "batiks," so called, on ebay and on the web called Bold Over Batiks. You can see pix of the kind of thing I'm interested in [in the picture to the right]. Do you mind telling me how you think these were done?
Bold Over Batiks is selling some beautiful fabrics. What the fabrics in that example from their eBay auction look most like is not batik at all, just as you suspected, but instead fabric that has been painted with fabric paints, stretched over a frame of some sort to temporarily hold it tight, and then large salt crystals have been dropped on the damp fabric paint.
The effect is reminiscent of raindrops. Now, I am not saying that it is impossible to somehow use batik wax to get a similar effect, but that's not what it looks like. What salt crystals do is draw the fabric paint toward them, as though to dilute the salt, by osmotic pressure. This sucks the paint away from surrounding areas. The technique works very well with pigment-based fabric paints. It will not work with fast-reacting dyes, such as Procion MX fiber reactive dyes. It can work with dyes that are very slow to set, however, in particular dyes that do not bond to the fiber until they are steamed. The issue is whether the dye or pigment attaches to the fabric before the salt has a chance to move it.
You can buy expensive little jars of 'silk salt' from the same suppliers that sell good silk paints, or for the same effect but far less expense you can buy any large-crystalled salt, such as kosher salt or the salts sold for deicing sidewalks or for use in water softeners. Salt effects work well with many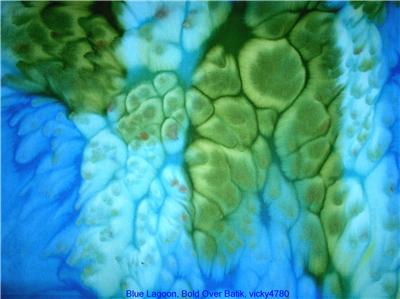 different
silk dyes and silk paints, such as the French silk dyes (including Tinfix, Pebeo
Soie, Dupont, and Ateliers Creatief Kniazeff), Dye-Na-Flow, SetaSilk, Marabu Silk Paint, and
Deka Silk Paint—probably also with any thin, transparent fabric paint.
They work best on tightly-stretched thin fabric. Salt effects will not work well
on coarse, thick weaves of fabric. The Dharma Trading Company catalog indicates
how well each of the Tinfix silk dye colors work for silk
effects.
different
silk dyes and silk paints, such as the French silk dyes (including Tinfix, Pebeo
Soie, Dupont, and Ateliers Creatief Kniazeff), Dye-Na-Flow, SetaSilk, Marabu Silk Paint, and
Deka Silk Paint—probably also with any thin, transparent fabric paint.
They work best on tightly-stretched thin fabric. Salt effects will not work well
on coarse, thick weaves of fabric. The Dharma Trading Company catalog indicates
how well each of the Tinfix silk dye colors work for silk
effects.

Another picture from the same vendor shows a different effect (see image above, to the left),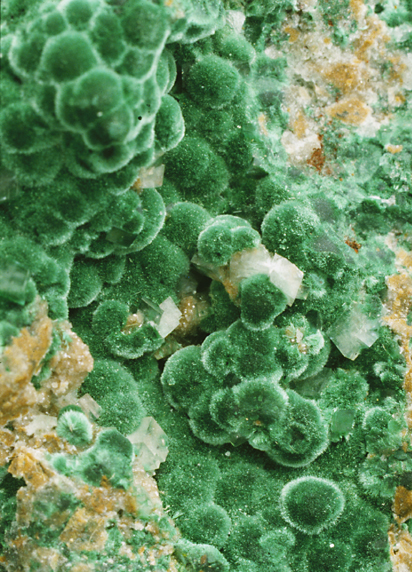 which
looks like what you might achieve by applying drops of silk paint to the fabric,
and then dropping alcohol on top. The alcohol pulls the dye with it as it creeps
along the fabric, leaving lighter areas. In this case the effect looks
wonderfully like the bulgy round crystals of natural rough malachite stones (the
picture at the right is from the Würzburg
Mineralogical Museum).
which
looks like what you might achieve by applying drops of silk paint to the fabric,
and then dropping alcohol on top. The alcohol pulls the dye with it as it creeps
along the fabric, leaving lighter areas. In this case the effect looks
wonderfully like the bulgy round crystals of natural rough malachite stones (the
picture at the right is from the Würzburg
Mineralogical Museum).
I used to use salt effects a lot when working with silk paint, back before I started using fiber reactive dyes. The results are really beautiful, and very easy to obtain. I think that you'll want to try silk painting so that you can experiment with salt and alcohol effects.
(Please help support this web site. Thank you.)


Message: I have read and combed over your website and had great fun with it, especially the low water immersion dyeing information. A zillion thank you's for your kind work.
I saw some "batiks," so called, on ebay and on the web called Bold Over Batiks. You can see pix of the kind of thing I'm interested in [in the picture to the right]. Do you mind telling me how you think these were done?
Bold Over Batiks is selling some beautiful fabrics. What the fabrics in that example from their eBay auction look most like is not batik at all, just as you suspected, but instead fabric that has been painted with fabric paints, stretched over a frame of some sort to temporarily hold it tight, and then large salt crystals have been dropped on the damp fabric paint.
The effect is reminiscent of raindrops. Now, I am not saying that it is impossible to somehow use batik wax to get a similar effect, but that's not what it looks like. What salt crystals do is draw the fabric paint toward them, as though to dilute the salt, by osmotic pressure. This sucks the paint away from surrounding areas. The technique works very well with pigment-based fabric paints. It will not work with fast-reacting dyes, such as Procion MX fiber reactive dyes. It can work with dyes that are very slow to set, however, in particular dyes that do not bond to the fiber until they are steamed. The issue is whether the dye or pigment attaches to the fabric before the salt has a chance to move it.
You can buy expensive little jars of 'silk salt' from the same suppliers that sell good silk paints, or for the same effect but far less expense you can buy any large-crystalled salt, such as kosher salt or the salts sold for deicing sidewalks or for use in water softeners. Salt effects work well with many
 different
silk dyes and silk paints, such as the French silk dyes (including Tinfix, Pebeo
Soie, Dupont, and Ateliers Creatief Kniazeff), Dye-Na-Flow, SetaSilk, Marabu Silk Paint, and
Deka Silk Paint—probably also with any thin, transparent fabric paint.
They work best on tightly-stretched thin fabric. Salt effects will not work well
on coarse, thick weaves of fabric. The Dharma Trading Company catalog indicates
how well each of the Tinfix silk dye colors work for silk
effects.
different
silk dyes and silk paints, such as the French silk dyes (including Tinfix, Pebeo
Soie, Dupont, and Ateliers Creatief Kniazeff), Dye-Na-Flow, SetaSilk, Marabu Silk Paint, and
Deka Silk Paint—probably also with any thin, transparent fabric paint.
They work best on tightly-stretched thin fabric. Salt effects will not work well
on coarse, thick weaves of fabric. The Dharma Trading Company catalog indicates
how well each of the Tinfix silk dye colors work for silk
effects.Another picture from the same vendor shows a different effect (see image above, to the left),
 which
looks like what you might achieve by applying drops of silk paint to the fabric,
and then dropping alcohol on top. The alcohol pulls the dye with it as it creeps
along the fabric, leaving lighter areas. In this case the effect looks
wonderfully like the bulgy round crystals of natural rough malachite stones (the
picture at the right is from the Würzburg
Mineralogical Museum).
which
looks like what you might achieve by applying drops of silk paint to the fabric,
and then dropping alcohol on top. The alcohol pulls the dye with it as it creeps
along the fabric, leaving lighter areas. In this case the effect looks
wonderfully like the bulgy round crystals of natural rough malachite stones (the
picture at the right is from the Würzburg
Mineralogical Museum). I used to use salt effects a lot when working with silk paint, back before I started using fiber reactive dyes. The results are really beautiful, and very easy to obtain. I think that you'll want to try silk painting so that you can experiment with salt and alcohol effects.
(Please help support this web site. Thank you.)
—ADVERTISEMENT—
