How can you tell to which class of acid dye a particular dye belongs?
Name: Zoe
—ADVERTISEMENTS—
Books about the chemistry of dyeing
Wool Dyeing
Edited by David M. Lewis, 1992.
Highly technical and thorough, this book explains all aspects of wool dyeing in a manner understandable by textile professionals and advanced science students.
Victor B. Ivanov's

Reactive Dyes in Biology and Medicine
Explains use of reactive dyes for staining proteins or carbohydrates
Waring and Hallas's

The Chemistry and Application of Dyes (Topics in Applied Chemistry)
includes recipes for synthesizing reactive dyes
Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
John Shore's

Cellulosies Dyeing
Useful information about the chemistry of reactive dyes, and other dye types used for cotton and other plant fibers
Country or region: Switzerland
Message: I would just like to ask a questions about the different types of acidic dyes: How do you tell that a dye is of which type? The dye in particular I am enquiring about specifically is Acid Blue 93. I think it is a acid-milling dye, but I am not sure. Thank you!
This is an interesting question. How can you tell to which class of acid dye a particular dye belongs? This question cannot be answered briefly.
The different acid dyes, not including the metal complex dyes, are classified into four different groups, rather arbitrarily, based not on any structural consideration, but instead on their wet fastness properties. From least washfast to most washfast, they are categorized as:
1. acid leveling dyes (also known as level-dyeing, strong acid, or equalizing acid dyes)
2. fast acid, half-milling, or perspiration-fast dyes
3. acid milling dyes
4. supermilling dyes
(From the 1992 book, Wool Dyeing, edited by David M. Lewis, in a chapter entitled "Dyeing wool with acid and chrome dyes", by P.A. Duffield.)
Acid milling dyes are named for possessing more wet fastness on textiles, during milling treatments, than some other groups of acid dyes. The optimal pH for their attachment to textile fibers is much higher than the pHs required for levelling dyes. While acid levelling dyes work best at a pH between 2.5 and 3.5, acid milling dyes work best at a much more mildly acid pH of 5, ranging all the way up to the very mildly basic pH of 8.
Experimental comparisons of wet fastness and optimal pH would be a slow and lengthy way to identify the classification of your acid dye. However, in this case, we're in luck: the molecular structure for Colour Index Acid Blue 93 is readily available online, on multiple sites. Let's see what we can make of it from its structure. Colour Index Acid Blue 93 is also known as Methyl Blue (not to be confused with Methylene Blue), Helvetia Blue, Ink Blue G, and Cotton blue, among others:
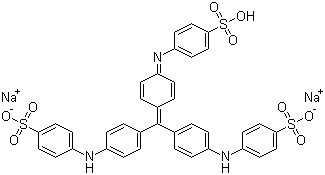
(from Chemblink.com, at http://www.chemblink.com/products/28983-56-4.htm)
It is also known as Colour Index number 42780, or, to give the full chemical name, as disodium [[4-[bis[4-[(sulphonatophenyl)amino] phenyl]methylene] cyclohexa-2,5-dien-1-ylidene] amino]benzenesulphonate. Its molecular weight is 799.8, and its CAS registry number (often useful in performing further searches) is 28983-56-4.
To start with, we can tell by looking at the structure that this is an acid dye, which means that it should be suitable for dyeing polyamide-based textile fibers, such as silk, wool, and nylon. (In spite of their name, acid dyes are not themselves strong acids, but instead are named for the usually mild acid used as an auxiliary when dyeing textiles with them.) We can see that this dye structure is not a fiber reactive dye, because there are no reactive components, such as a dichlorotriazine section. It's not a basic (cationic) dye, because, without the associated sodium ions, it has a negative charge; basic dyes have positive molecular charges. It's not a vat dye, both from the obvious fact that it's a salt, rather than being neutral in charge, and because it lacks ketone (C=O) groups, which are part of the structure characteristic of vat dyes. Unlike a direct dye, it is not long, narrow, and planar in shape; the aryl groups are twisted, instead, creating a propeller shape (as cited by Dean Thetford of Zeneca Specialities in 2000 in a piece entitled "Triphenylmethane and Related Dyes" that appeared online in the Kirk-Othmer Encyclopedia of Chemical Technology and in Van Nostrand's Encyclopedia of Chemistry). There are no carboxylic groups adjacent to hydroxyl groups, as you may see in mordant dyes. It's obviously not a metal complex dye (also known as a pre-metalized dye), because there are no heavy metals indicated in the structure at all. Like other acid dyes, it contains sulfonate groups, which help to make it water-soluble. It's interesting to note that some dyes that belong to other classes, such as fiber reactive dyes, include these sulfonate groups, too, and are therefore, under the correct circumstances, capable of acting as acid dyes. (See "What kinds of chemical bonds attach dyes to fibers?".)
Looking at its chemical structure, the chromophore (color producing section) of this dye is clearly of the triarylmethane type, resembling the chemical triphenylmethane. Acid dyes having structures related to triphenylmethane are said to predominate in the milling class of dye, as mentioned in Wikipedia since 2006, but I don't see a proper reference for this claim there; much more recently, this statement has appeared, word-for-word but without proper attribution, in Dharma Trading Company's useful new page on how acid dyes work. I'm not quite sure whether the statement is intended to claim that most triphenylmethane dyes as acid milling dyes, or that most acid milling dyes are triphenylmethanes; this particular Wikipedia article is not written to as high a standard as many other Wikipedia entries. A number of dyes with similar triarylmethane chromophores, but lacking the sulfonate groups, are found among the basic dyes, including some of the first dyes synthesized, such as crystal violet. Triphenylmethane dyes are noted for poor lightfastness.
That said, the surest way I know to determine whether an acid dye is in the group known as acid milling dyes is to find it listed as such for sale. I have not myself been able to find Colour Index Acid Blue 93 listed as an acid milling dye, or as a leveling acid dye, or any other specific category of acid dye. It does not appear to be much used in textiles, but instead appears to be manufactured mainly for use in inks, in addition to its use as a biological stain. Many acid milling dyes are listed as such by their manufacturers; for example, Town End Colours for Industry has a page listing Acid Milling Dyes for Nylon and Other Polyamides, not including C. I. Acid Blue 93. It's not included in the listing of acid milling dyes for any manufacturer I have looked at. Although PRO Chemical & Dye's and Maiwa's lines of WashFast Acid Dyes (formerly made by Ciba) comprise mostly fast milling dyes, it is not included among them, either (see my page, "Which Washfast Acid colors are pure, and not mixtures?"), nor is it included among the Jacquard Acid Dyes. In fact, Acid Blue 93 does not appear to be available as a textile dye from any of the suppliers of textile dyes for hand dyers. It looks as though it is best obtained from a supplier of biological stains, such as Sigma Aldritch.
(Please help support this web site. Thank you.)
There's no obvious structural difference between a leveling acid dye versus an acid milling dye, since acid dyes are classified according to fastness rather than by structure, but the size of the molecule is itself a good indication. Smaller molecules are better at leveling, but are less wet fast; larger acid dye molecules are worse at leveling and better at wet fastness. The relative molecular weight of this dye is typical of an acid milling dye. Its relative molecular weight is listed in various online sources as 799.8. According to David M. Lewis's book, Wool Dyeing, the relative molecular weights of acid milling dyes range from 600 to 900. In contrast, the relative molecular weights of acid leveling dyes, also known as strong acid dyes, range from 300 to 500 for the monosulfonated dyes, and 400 to 600 for the disulfonated ones. (Almost all levelling acid dyes contain either one or two sulfonate groups.) The fast acid dyes, also known as half-milling or perspiration-fast dyes, range from 500 to 600 relative molecular mass and are typically monosulfonated. So, just looking at the size of the molecule, we can be pretty sure that Acid Blue 93 is an acid milling dye, though we can't distinguish it from a supermilling dye.
(Please help support this web site. Thank you.)
Posted: Thursday - August 04, 2011 at 09:16 AM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:49 PM
Total entries in this category:
Published On: Aug 29, 2012 02:49 PM
