Are the bonds that connect dye and fabric polar covalent or non-polar covalent?
Question: Are the bonds that connect dye and fabric polar covalent or non-polar covalent?
—ADVERTISEMENT—

Linda Knutson's
Synthetic Dyes for Natural Fibers
Heinrich Zollinger
Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments
The type of bond between dye and fiber depends on the type of dye. Different classes of dye attach to fibers via quite different forms of bonding. Most types of dye do not form covalent bonds to the textile fiber.
Fiber reactive dyes are the only dyes that form covalent bonds with the fiber, effectively becoming one molecule with it. Before the dye reaction, the reactivity of the fiber reactive dye molecule relies on polar covalent bonds.
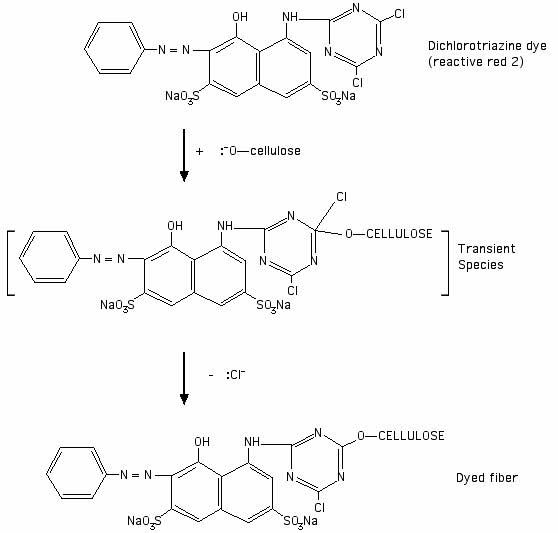
Using a dichlorotriazine dye (Procion MX dye) as an example: before the dye reacts with the fiber, the dye structure contains two chlorine atoms. The electronegativity of the chlorine atoms makes the dye susceptible to nucleophilic attack on the adjacent carbon atoms. Cellulose is activated to the nucleophilic cellulosate anion form by a high pH, as in the presence of soda ash, and then attacks one of the carbons that is attached to a halogen ion. See the drawing below as an illustration of the reaction:
[Drawing based on an illustration of a generic dichlorotriazine reaction in Chapter 4 of John Shore's 1995 book Cellulosics Dyeing, published by the Society of Dyers and Colorists.]
After the reaction has occurred, the halogen atom is replaced by the cellulosate. The carbon on the ring structure is attached to a nitrogen on either side in the ring within the dye itself, and to the oxygen of the cellulose molecule. From your studies so far, you should now have enough information to decide whether the covalent bond between the cellulose and the carbon atom to which it is attached is polar or non-polar, based on the respective electronegativities of carbon and oxygen atoms.
Most dyes do not form any sort of covalent bond with the fiber, whether polar or non-polar. Look at my page, "What kinds of chemical bonds attach dyes to fibers?". The only covalent bonding between dye and fiber occurs in the case of the fiber reactive dyes. These dyes are typically used for cellulose fibers, though they can also be used on protein fibers.
Acid dyes, which are normally the preferred choice of dyes for protein fibers, as well as for nylon, are attached to the fiber through hydrogen bonding and salt linkages. Direct dyes, which are the most inexpensive dyes available for plant-based fibers, loosely associate with the fiber through the dye property called substantivity, which relies on Van der Waals forces and some hydrogen bonding. Disperse dyes, which are used for synthetic fibers such as polyester and acetate, are dispersed through the fiber by vaporization from high heat, and are not bonded to it at all. Vat dyes, most popularly including the synthetic indigo used to dye blue denim, are converted to a soluble form and allowed to lodge inside the fibers before being returned to an insoluble form; naphthol dyes are held in the fiber by insolubility in much the same way. Mordant dyes, including most natural dyes, do not attach directly to the fiber at all, but instead form a complex to a metal ion which forms a bridge between the dye and the fiber. For more details, see my page, "What kinds of chemical bonds attach dyes to fibers?".
Please be careful to properly cite your sources in your schoolwork, as in the following citation for this article:
Burch, Paula. "Are the Bonds That Connect Dye and Fabric Polar Covalent or Non-polar Covalent?"Paula Burch's All About Hand Dyeing. 11 January 2011. <http://www.pburch.net/dyeing/dyeblog/C1845207367/E20110111074828/index.html>(Please help support this web site. Thank you.)
Posted: Tuesday - January 11, 2011 at 07:48 AM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:49 PM
Total entries in this category:
Published On: Aug 29, 2012 02:49 PM