What kinds of chemical bonds attach basic dyes to fabrics?
Name: Ravinder
Message: What kinds of chemical bonds attach basic dyes to fabrics?
Message: What kinds of chemical bonds attach basic dyes to fabrics?
—ADVERTISEMENTS—
Methylene Blue is a Basic dye—ADVERTISEMENTS—
Books About the Chemistry of Textile DyesThis is an interesting question to consider. The one feature that distinguishes basic dyes from all other dyes is their ionic charge. The molecules of basic dyes have a positive charge, in contrast to vat dyes , which carry a neutral charge (that is, no charge at all), and all other dyes, including reactive dyes, acid dyes, and direct dyes, which carry a negative charge.
Since the fibers used in fabrics are negatively charged, the positively charged basic dye molecules are attracted to the fibers.
Basic dyes can be used to dye natural fibers such as wool, but their lightfastness on these materials is so poor that other dyes are much preferred. However, basic dyes are commonly used to dye acrylic fiber, which does not present the same light-fading problem. Acrylic fiber cannot be dyed with most classes of dye, such as reactive or acid dyes. Although acrylic fiber can be dyed with disperse dye, only light to medium shades may be obtained, so basic dyes are essential in order to obtain brilliant or dark colors on acrylic fiber.
Many other substances share the negative charge of textiles fibers. This means that basic dyes tend to stick to almost anything they touch. Basic dyes are inconvenient for home-dyers to use, because they stain anything they touch, such as a countertop or a plastic dishpan. Other dyes are easily washed from plastic materials after a splash.
Because of their positive charge, basic dyes are also known as cationic dyes. You can tell whether a written dye structure is a basic dye at a glance, by looking for the positive charge indication on the molecular structure. Here is a drawing of methylene blue, or basic blue 9, as an example:
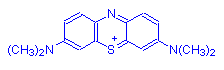
In my PhD work, I found that methylene blue intercalated into DNA, inserting itself into the ladder-like structure of the DNA molecule, where it would react with light and oxygen to produce breakage of the DNA. It's worth noting that anything that breaks DNA is likely to cause mutations and cancer. I do not recommend the use of basic dyes outside of a properly equipped laboratory, where they must be used with appropriate safety precautions.
(Please help support this web site. Thank you.)
Posted: Wednesday - January 14, 2009 at 07:40 AM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM