I'm wondering if you could explain the chemistry behind why cotton can't be dyed at an acidic pH.
Name: Lulu
Message: Hello, I'm wondering if you could explain the chemistry behind why cotton can't be dyed at an acidic pH. I did an experiment at school where I used 15 mL of HCl to dye a 100% cotton cloth using the Procion MX dye (fuschia) and the colour absorbed by the cotton appeared the same as the one with a pH of 8. The concentration of HCl was 1 mol/L. Thanks
At a pH of 8, I see quite a bit of reaction between Procion MX dye and the fiber, though much less than at a pH of 9 or 10, but I see essentially none at pH 7 and below. The small amount of HCl (hydrochloric acid) that you used probably produced a pH just below 7, but if your water supply is very alkaline the final pH may still have been above 7. (You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.
(You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.
Cotton fibers are made of long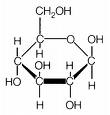 molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.
molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.
In the picture above, the carbons are shown with the letter "C", but in most chemical drawings, there are so many carbons that they are not drawn at all; wherever two lines meet at an angle, if there is no other letter written there, you can assume that that indicates that there is a carbon atom at the junction of two lines. Here is a drawing of a section of a cellulose molecule, showing how the glucose units are connected together to form the cellulose chain: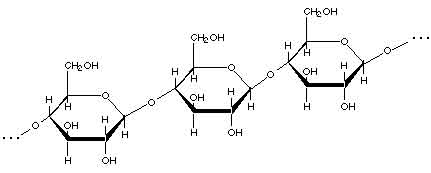
(The darker lines are supposed to make the molecule look more three-dimensional; you can ignore them for now.)
The way that cellulose reacts with fiber reactive dye, such as Procion MX dye, is shown in the drawing below, from the May 19, 2005 posting in this blog, "Chemical reaction for a dichlorotriazine dye with cellulose". The large molecule shown is the dye molecule, while the cellulose chain is represented by the word "cellulose", in this drawing:
Before the cellulose can react, it must first become activated by losing a hydrogen ion to become what is called a cellulosate anion, with a negative charge. After the cellulose has lost its hydrogen ion, it can attack the carbon adjacent to one of the chlorines in the dye molecule. (The chlorine makes that carbon atom more electronegative than any of the carbons that do not have a chlorine atom attached to them, so it is somehow more vulnerable to attack.) In the presence of a high pH, such as is provided by soda ash or sodium hydroxide, the cellulose readily loses that hydrogen in order to become a cellulosate anion. Think of it this way: at a pH above neutral, there are, in effect, extra hydroxyl ions (OH) floating around in the water. With more free hydroxyls, that hydrogen on the cellulose is more easily stolen away to make a new water molecule, with two hydrogens and one oxygen. However, when you add acid, such as the HCl you used, this reduces the number of available OHs floating around in the water in your dyebath.
After the cellulose is ionized by the loss of that hydrogen atom, it readily reacts with the fiber reactive dye. The dye shown in the drawing above is a dichlorozine or Procion MX dye, red MX-5B, also known as Colour Index reactive red 2. The reaction is similar for other fiber reactive dyes; for example, see the drawing in the posting in this blog for February 21, 2006. You can compare the chemical structure of your fuchsia Procion MX dye (which is red MX-8B, or reactive red 11) on my page "What is the chemical structure of Procion MX dye?", at http://www.pburch.net/dyeing/FAQ/structure.shtml . The two dyes are very similar in structure and in function.
However, in contrast, the "reaction" between a different class of dye, direct dye, with cotton is completely different. Direct dye is the fast-fading dye found in all-purpose dyes, such as Rit dye or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.
or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.
(Please help support this web site. Thank you.)
Message: Hello, I'm wondering if you could explain the chemistry behind why cotton can't be dyed at an acidic pH. I did an experiment at school where I used 15 mL of HCl to dye a 100% cotton cloth using the Procion MX dye (fuschia) and the colour absorbed by the cotton appeared the same as the one with a pH of 8. The concentration of HCl was 1 mol/L. Thanks
At a pH of 8, I see quite a bit of reaction between Procion MX dye and the fiber, though much less than at a pH of 9 or 10, but I see essentially none at pH 7 and below. The small amount of HCl (hydrochloric acid) that you used probably produced a pH just below 7, but if your water supply is very alkaline the final pH may still have been above 7.
 (You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.
(You
should have used pH paper to test what the pH of a solution of your water with
that amount of HCl would produce, without adding the dye until after checking
the pH since dye makes it impossible to read the color of the pH indicator
paper.) The cotton fabric will be stained at any pH, but then the dye comes out
in the wash if it has not properly reacted with the fiber. Rinse the fabric
first in cool water to remove soda ash and any other auxiliary chemicals, then
wash in very hot water, anywhere from 140°F (60°C) to boiling, to
remove the excess unattached dye.Cotton fibers are made of long
 molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.
molecules called cellulose, which are chains of glucose sugar molecules hooked
together in a particular way that is indigestible to humans and makes it resist
rapid microbial degradation.(Starch is another kind of chain of glucose
molecules, but we have enzymes that can digest starch into its individual sugar
units.) The individual glucose molecules have hydroxyl groups sticking out of
them, drawn as -OH groups. Here, to the right, is a drawing of a glucose
molecule.In the picture above, the carbons are shown with the letter "C", but in most chemical drawings, there are so many carbons that they are not drawn at all; wherever two lines meet at an angle, if there is no other letter written there, you can assume that that indicates that there is a carbon atom at the junction of two lines. Here is a drawing of a section of a cellulose molecule, showing how the glucose units are connected together to form the cellulose chain:

(The darker lines are supposed to make the molecule look more three-dimensional; you can ignore them for now.)
The way that cellulose reacts with fiber reactive dye, such as Procion MX dye, is shown in the drawing below, from the May 19, 2005 posting in this blog, "Chemical reaction for a dichlorotriazine dye with cellulose". The large molecule shown is the dye molecule, while the cellulose chain is represented by the word "cellulose", in this drawing:

Before the cellulose can react, it must first become activated by losing a hydrogen ion to become what is called a cellulosate anion, with a negative charge. After the cellulose has lost its hydrogen ion, it can attack the carbon adjacent to one of the chlorines in the dye molecule. (The chlorine makes that carbon atom more electronegative than any of the carbons that do not have a chlorine atom attached to them, so it is somehow more vulnerable to attack.) In the presence of a high pH, such as is provided by soda ash or sodium hydroxide, the cellulose readily loses that hydrogen in order to become a cellulosate anion. Think of it this way: at a pH above neutral, there are, in effect, extra hydroxyl ions (OH) floating around in the water. With more free hydroxyls, that hydrogen on the cellulose is more easily stolen away to make a new water molecule, with two hydrogens and one oxygen. However, when you add acid, such as the HCl you used, this reduces the number of available OHs floating around in the water in your dyebath.
After the cellulose is ionized by the loss of that hydrogen atom, it readily reacts with the fiber reactive dye. The dye shown in the drawing above is a dichlorozine or Procion MX dye, red MX-5B, also known as Colour Index reactive red 2. The reaction is similar for other fiber reactive dyes; for example, see the drawing in the posting in this blog for February 21, 2006. You can compare the chemical structure of your fuchsia Procion MX dye (which is red MX-8B, or reactive red 11) on my page "What is the chemical structure of Procion MX dye?", at http://www.pburch.net/dyeing/FAQ/structure.shtml . The two dyes are very similar in structure and in function.
However, in contrast, the "reaction" between a different class of dye, direct dye, with cotton is completely different. Direct dye is the fast-fading dye found in all-purpose dyes, such as Rit dye
 or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.
or Tintex Easy Fabric dye. It does not react with cellulose in the same way as a
fiber reactive dye. Instead, it bonds
relatively loosely to the cellulose, which is why it is so much more impermanent
and easy to wash out with hot water. This loose association between direct dye
and cellulose does not require a high pH, because there is no reaction like that
between Procion MX dye and cellulose. Direct dyes require only simmering water
and, optionally, salt, to attach in their weak way to the cellulose, since they
do not form the strong covalent bonds illustrated in the reaction picture above.
However, since the direct dye does not form strong bonds to the cellulose, its
performance as a dye is unsatisfactory unless it is fixed in place with a
special dye fixative, that is, a cationic dye fixative
such as Retayne, a product which sticks to the dye molecules through
ionic attraction, since Retayne particles have a positive charge and most dye
particles have a negative charge; the particles of Retayne essentially glue the
molecules of direct dye in place, making it acceptably washfast, though never as
good as a fiber reactive dye. It is only the strong covalent bond that connects
fiber reactive dye to cellulose that is truly permanent.(Please help support this web site. Thank you.)
Posted: Monday - December 31, 2007 at 10:01 AM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM