the chemical, biological and physical processes and properties that generate the colours in clothing
Name: Barry
Message: hi,
I have really enjoyed your site. I am an undergrad student in Toronto, Canada, and I'm in a science course, 'Understanding Colour'. I am wondering if you have some ideas for web and non-web resources for a project I am working on?
My topic is Colours in Clothing (dyes). We have been asked to research the chemical, biological and physical processes and properties that generate the colours. I have found many books in our science library but they require a more advanced understanding of organic chemistry than I have, and I would like to start off with a clear understanding of the major ideas and concepts. Do you have any suggestions?
I have read your lesson plan and I really enjoyed it. I need more sources which are as accessible as yours.
Unfortunately, I don't know of other equally accessible sources, printed or online; this is one of my major motivations in writing the articles on my web site. Books on dyeing in general either show the chemistry and are impenetrable to beginners, or ignore the chemical structures altogether. I want to make the chemistry of dyeing more accessible and interesting to people who don't already understand the language of the technical information that is available.
Here is the most important point to know about dyes: there are two different aspects to how a dye works. One is how it attaches to the fiber, and the other is how it makes the color. These two properties may be provided by entirely different parts of the dye molecule, though this is not always the case. A third property, solubility in water, is often provided by the presence of sulfonate groups on the dye molecule.
For example, in the case of reactive dyes, there is one section of the dye molecule that is called the chromophore; this provides the color of the dye. Each molecule of reactive dye also includes a section known as the reactive section; this is the part that actually reacts with the cellulose or protein fiber in order to form a permanent covalent bond.
The other common type of dye for cellulose fibers is called direct dye. These are large molecules that do not have a reactive section. The colored section of the molecule is all that there is. Its size helps it to loosely associate with the cellulose fiber, though higher temperatures tend to break these weak attractions and wash out the dye.
Protein fibers, such as silk and wool, are more complicated, since they are made of repeats of some twenty amino acids, instead of repeats of a single sugar subunit as in the case of cellulose.
For more information on how different types of dyes attach to clothing fibers, see my page, "What kinds of chemical bonds attach dyes to fibers? ".
The question of how different dye molecules make the different colors is much more complex and difficult to understand than the question of how dyes attach to clothing fibers. If you look at drawings of the structures of a number of different dyes, you will see that most of them contain multiple aromatic ring structures, or at least alternating single and double bonds. Certain arrangements of these cause a molecule to absorb specific wavelengths of light. When a molecule absorbs light in the visible region of the electromagnetic spectrum, the remaining wavelengths that are reflected from the substance give it its color. If red light is absorbed and other light reflected, you get the appearance of green. If blue is absorbed, you see orange; if violet is absorbed, you see yellow (which may be a mixture of red, orange, yellow, and green light); if yellow is absorbed, you see purple (which is a combination of red and blue wavelengths together.
A website called Stainsfile contains a worthwhile discussion about what makes different molecules have color, which you will want to read.
Specific colors tend to be created by similar structures among different classes of dye. For example, almost all turquoise blue dyes are based on a copper phthalocyanine ring, with various chemical groups attached to give the dye the ability to react with cotton, or act as a direct dye, or an acid dye for protein fibers, etc. You can see a picture of the structure for Color Index Reactive Blue 140, Procion turquoise MX-G, on the left; it is an enormous ring structure with a copper atom in the middle. This dye is actually a mixture of several closely related molecules, with either two or three reactive groups and one or two sulfonate groups attached at any of several points of the phthalocyanine ring.
Dr. Steve Mihok has a wonderful summation of different chromophores in all sorts of blue dyes on his site. Blue dyes can contain any of several different chromophores, including anthraquinones and triphenodioxazines. The triphenodioxazine structure contains five adjacent rings, including two nitrogens and two oxygens in the rings. Anthraquinone, the parent of the anthraquinone dyes, consists of a triple ring structure with two oxygen atoms attached to the middle ring. Indigo, the only widely-used good natural blue dye, though now mainly used in its synthetic form, gives its name to the indanthrone dyes. One way that purple can be produced is found in a close chemical relative of indigo, 6,6'dibromoindigo, which is the famous dye of antiquity known as Tyrian Purple.
Anthraquinone structures can also produce an orange or red color, depending on the attached substituent groups. The most common chromophores for red, orange, and yellow dyes are azo dye structures.
The best basic book I know of for beginners in this topic is
 Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.
Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.
(Please help support this web site. Thank you.)
Message: hi,
I have really enjoyed your site. I am an undergrad student in Toronto, Canada, and I'm in a science course, 'Understanding Colour'. I am wondering if you have some ideas for web and non-web resources for a project I am working on?
My topic is Colours in Clothing (dyes). We have been asked to research the chemical, biological and physical processes and properties that generate the colours. I have found many books in our science library but they require a more advanced understanding of organic chemistry than I have, and I would like to start off with a clear understanding of the major ideas and concepts. Do you have any suggestions?
I have read your lesson plan and I really enjoyed it. I need more sources which are as accessible as yours.
Unfortunately, I don't know of other equally accessible sources, printed or online; this is one of my major motivations in writing the articles on my web site. Books on dyeing in general either show the chemistry and are impenetrable to beginners, or ignore the chemical structures altogether. I want to make the chemistry of dyeing more accessible and interesting to people who don't already understand the language of the technical information that is available.
Reactions of various reactive dyes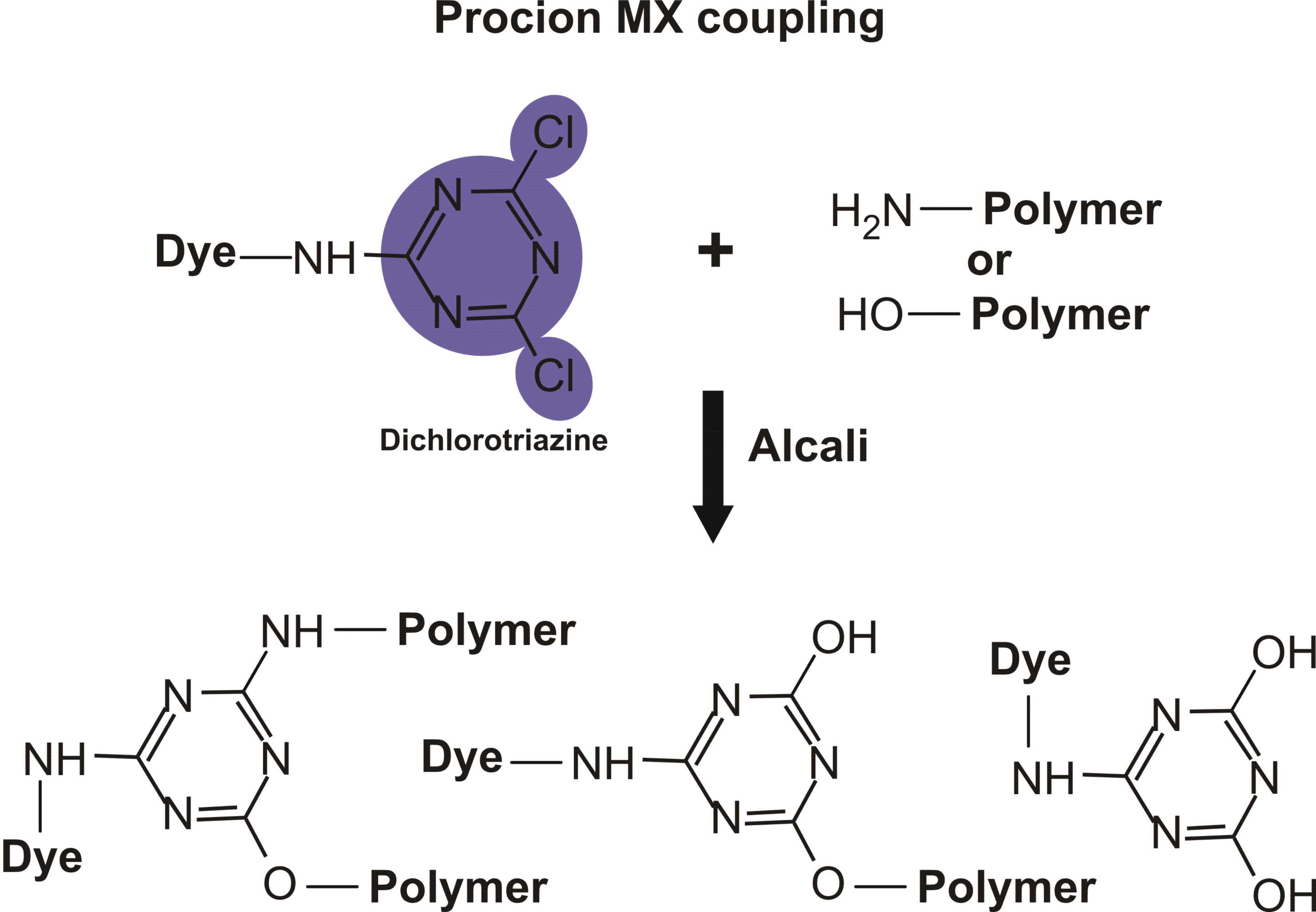
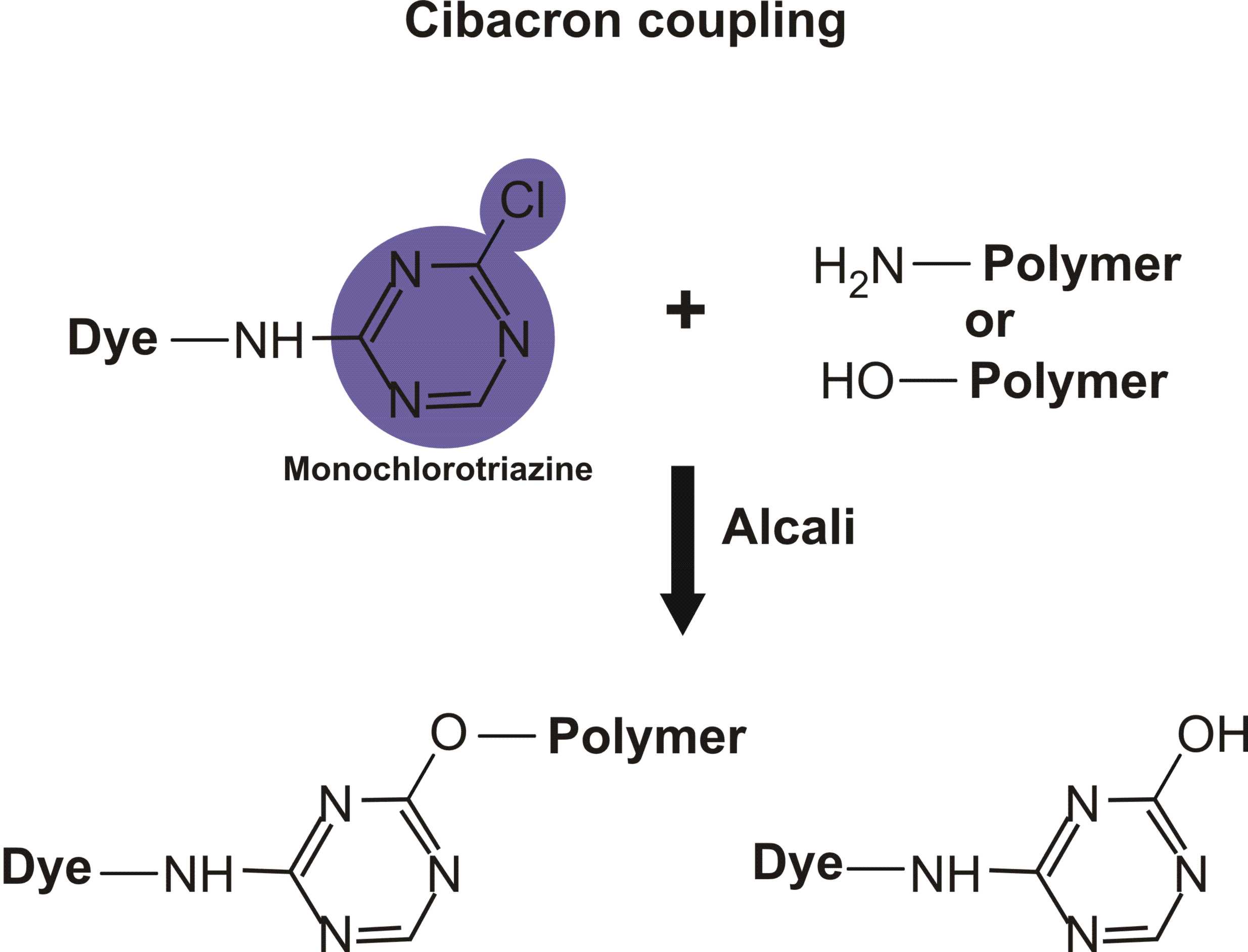
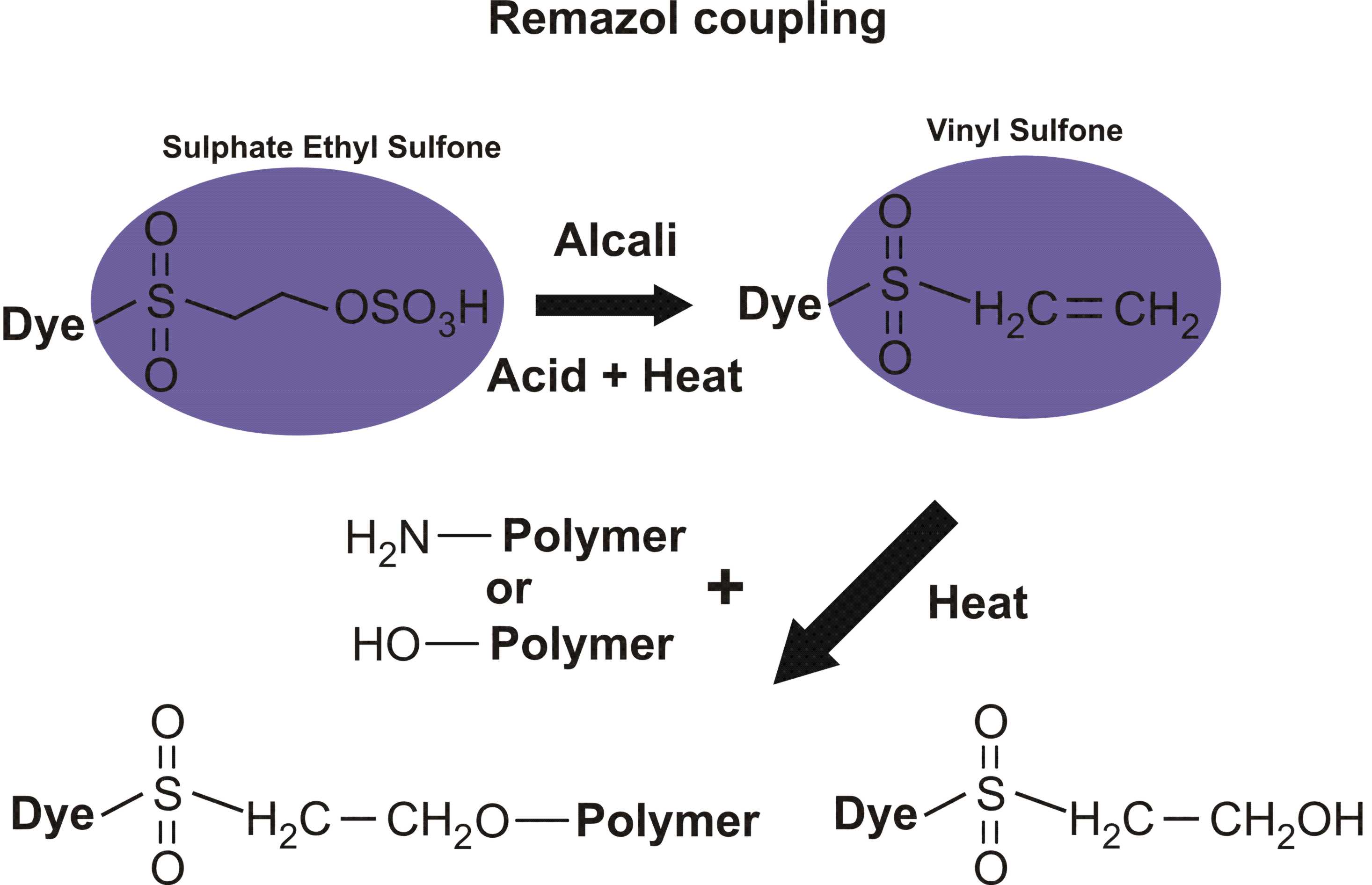



Structures of various reactive dyes
(chromophore is shown on the left side, reactive group on the right)
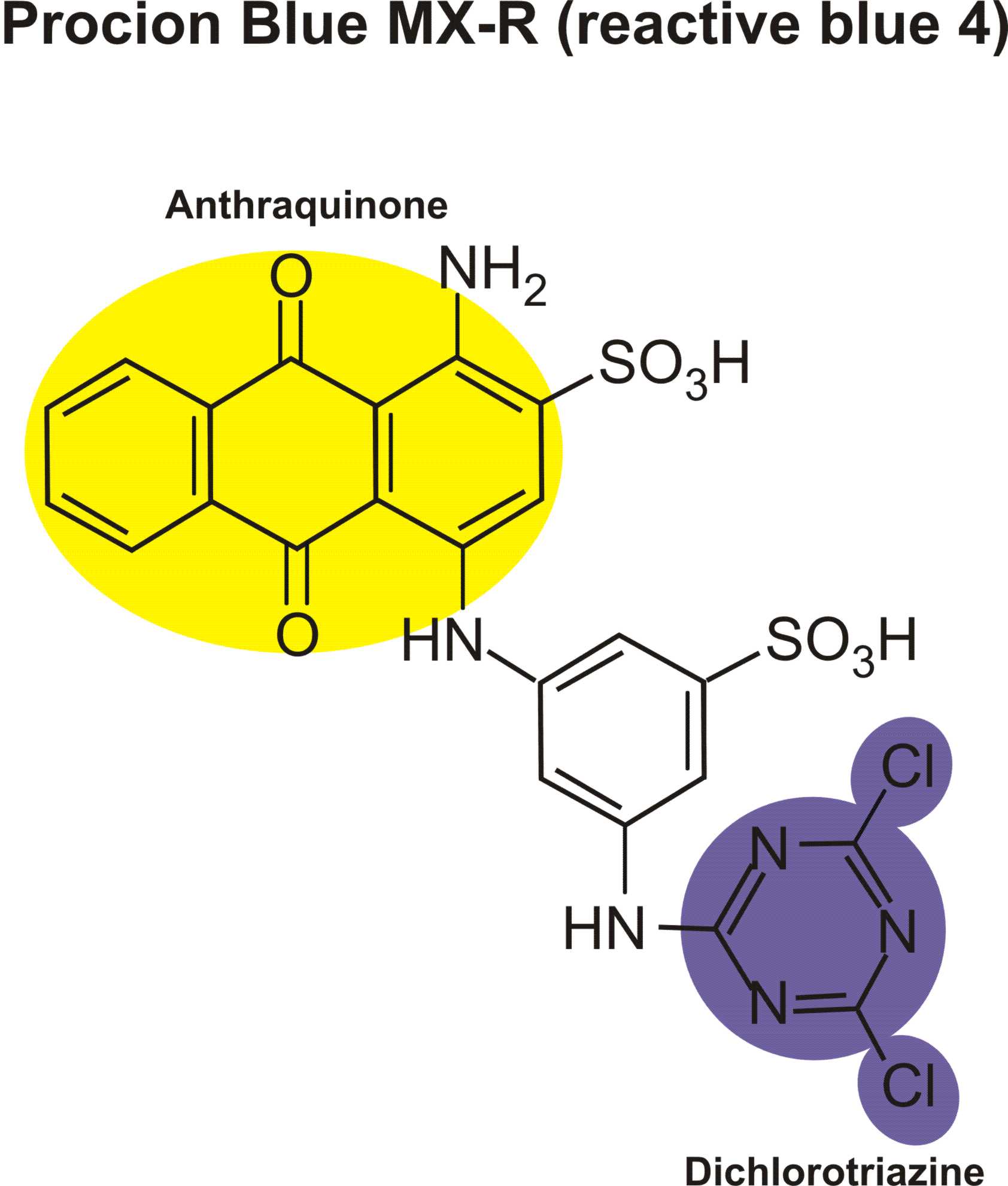
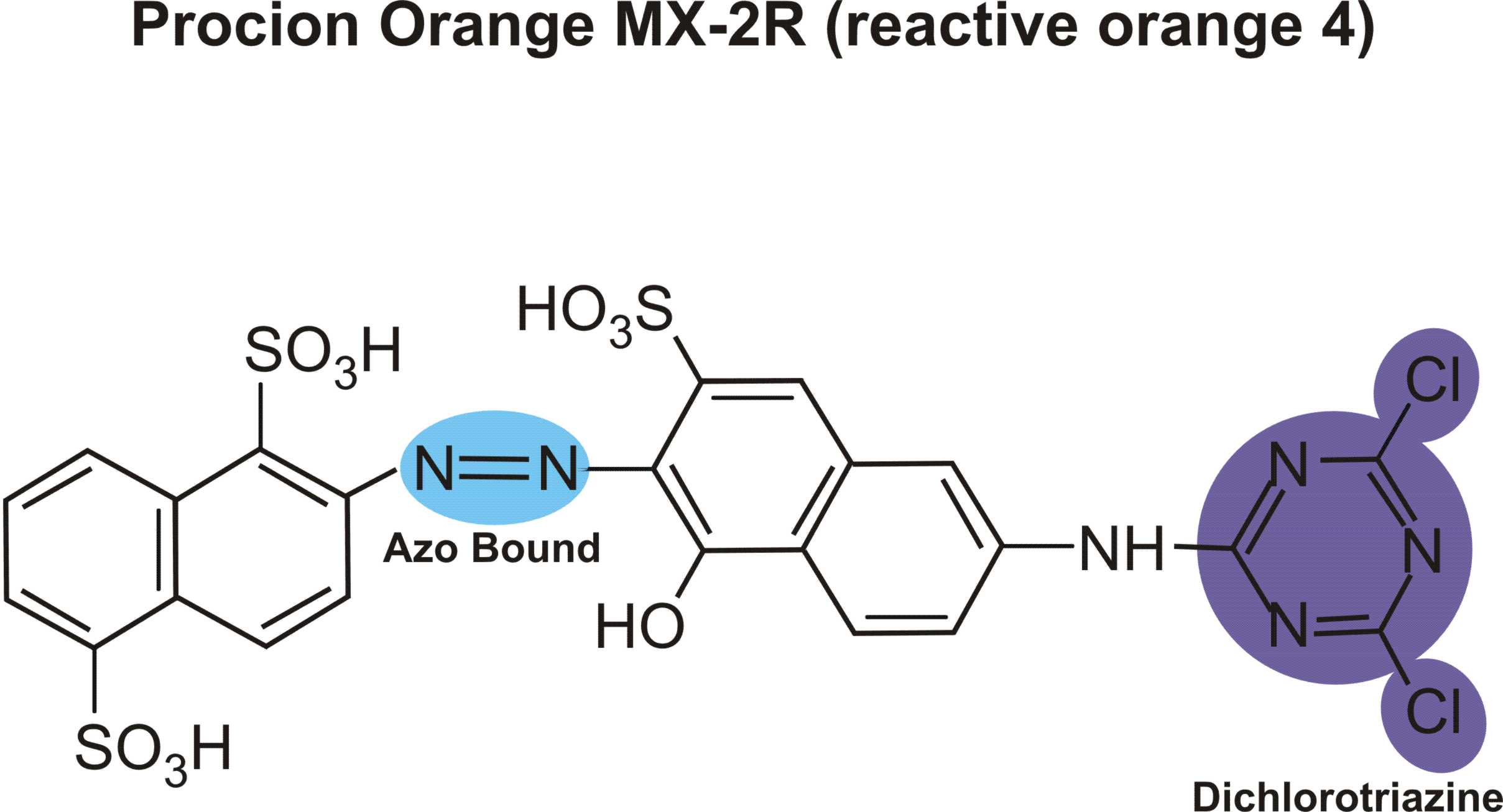
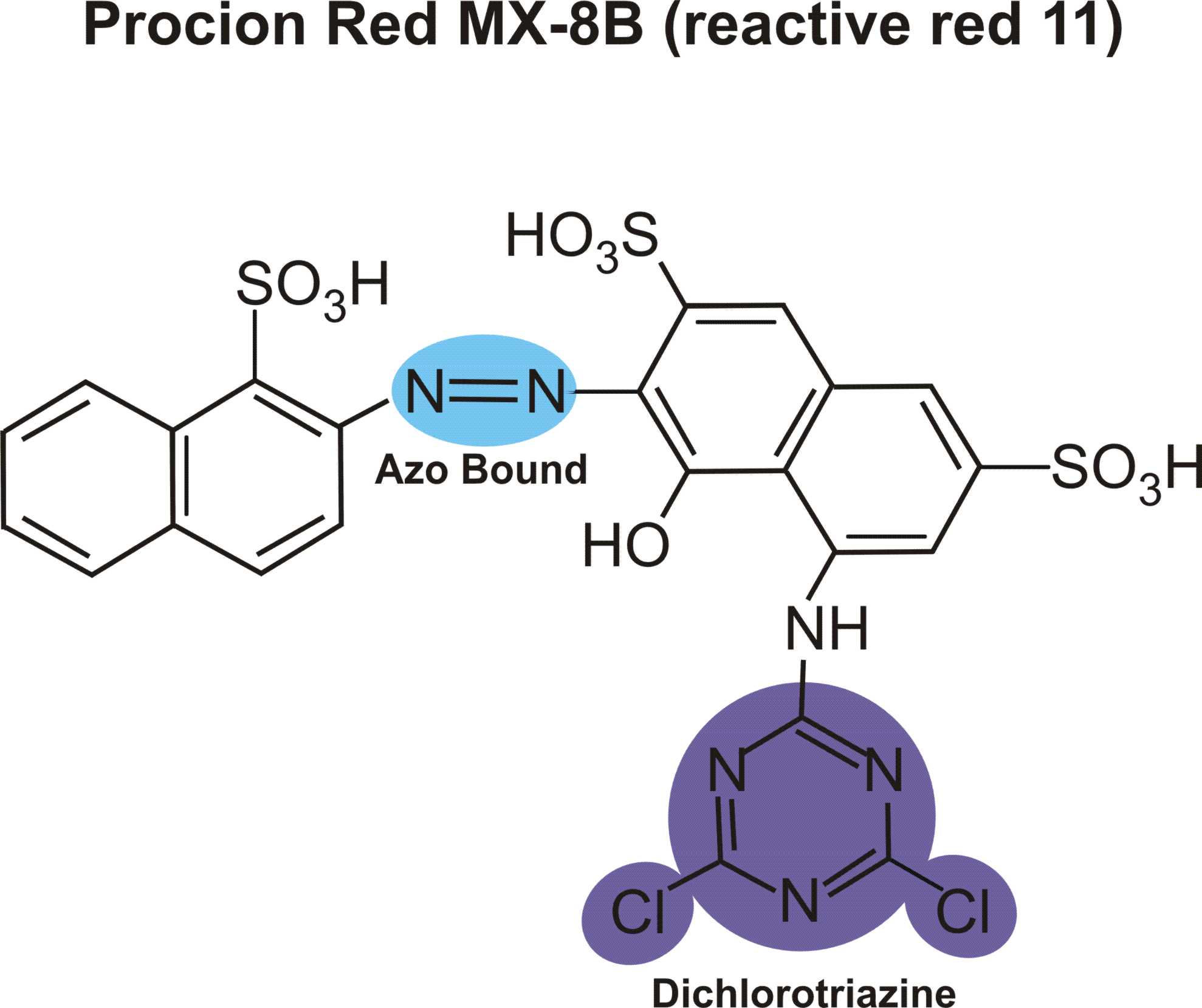
(The illustrations shown above, with the chromophore shown in yellow or blue and the reactive group shown in violet, were drawn by Dr. Francois Denis, who kindly gave permission for me to use them for educational purposes.)
(chromophore is shown on the left side, reactive group on the right)



(The illustrations shown above, with the chromophore shown in yellow or blue and the reactive group shown in violet, were drawn by Dr. Francois Denis, who kindly gave permission for me to use them for educational purposes.)
Here is the most important point to know about dyes: there are two different aspects to how a dye works. One is how it attaches to the fiber, and the other is how it makes the color. These two properties may be provided by entirely different parts of the dye molecule, though this is not always the case. A third property, solubility in water, is often provided by the presence of sulfonate groups on the dye molecule.
For example, in the case of reactive dyes, there is one section of the dye molecule that is called the chromophore; this provides the color of the dye. Each molecule of reactive dye also includes a section known as the reactive section; this is the part that actually reacts with the cellulose or protein fiber in order to form a permanent covalent bond.
The other common type of dye for cellulose fibers is called direct dye. These are large molecules that do not have a reactive section. The colored section of the molecule is all that there is. Its size helps it to loosely associate with the cellulose fiber, though higher temperatures tend to break these weak attractions and wash out the dye.
Protein fibers, such as silk and wool, are more complicated, since they are made of repeats of some twenty amino acids, instead of repeats of a single sugar subunit as in the case of cellulose.
For more information on how different types of dyes attach to clothing fibers, see my page, "What kinds of chemical bonds attach dyes to fibers? ".
The question of how different dye molecules make the different colors is much more complex and difficult to understand than the question of how dyes attach to clothing fibers. If you look at drawings of the structures of a number of different dyes, you will see that most of them contain multiple aromatic ring structures, or at least alternating single and double bonds. Certain arrangements of these cause a molecule to absorb specific wavelengths of light. When a molecule absorbs light in the visible region of the electromagnetic spectrum, the remaining wavelengths that are reflected from the substance give it its color. If red light is absorbed and other light reflected, you get the appearance of green. If blue is absorbed, you see orange; if violet is absorbed, you see yellow (which may be a mixture of red, orange, yellow, and green light); if yellow is absorbed, you see purple (which is a combination of red and blue wavelengths together.
A website called Stainsfile contains a worthwhile discussion about what makes different molecules have color, which you will want to read.
Specific colors tend to be created by similar structures among different classes of dye. For example, almost all turquoise blue dyes are based on a copper phthalocyanine ring, with various chemical groups attached to give the dye the ability to react with cotton, or act as a direct dye, or an acid dye for protein fibers, etc. You can see a picture of the structure for Color Index Reactive Blue 140, Procion turquoise MX-G, on the left; it is an enormous ring structure with a copper atom in the middle. This dye is actually a mixture of several closely related molecules, with either two or three reactive groups and one or two sulfonate groups attached at any of several points of the phthalocyanine ring.
Dr. Steve Mihok has a wonderful summation of different chromophores in all sorts of blue dyes on his site. Blue dyes can contain any of several different chromophores, including anthraquinones and triphenodioxazines. The triphenodioxazine structure contains five adjacent rings, including two nitrogens and two oxygens in the rings. Anthraquinone, the parent of the anthraquinone dyes, consists of a triple ring structure with two oxygen atoms attached to the middle ring. Indigo, the only widely-used good natural blue dye, though now mainly used in its synthetic form, gives its name to the indanthrone dyes. One way that purple can be produced is found in a close chemical relative of indigo, 6,6'dibromoindigo, which is the famous dye of antiquity known as Tyrian Purple.
Anthraquinone structures can also produce an orange or red color, depending on the attached substituent groups. The most common chromophores for red, orange, and yellow dyes are azo dye structures.
The best basic book I know of for beginners in this topic is

 Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.
Linda
Knutson's Synthetic Dyes for Natural Fibers. It is out of print but often
available for reasonable prices from online used books suppliers. It mentions
only a little chemistry, but is quite reliable. Another book suitable for
students in textile dyeing, a book that mentions how different dye chemicals
make the colors that they do, is Giles's Laboratory Course in Dyeing, by
David G Duff and Roy S. Sinclair. I have the fourth edition, 1989, which I
mail-ordered directly from the Society of Dyers and Colourists in the UK for
£9. It gives some idea of what chemical structures are responsible for what
colors. It's not as easy to understand as that lesson plan that I wrote for an
elementary school class, however.(Please help support this web site. Thank you.)
Posted: Wednesday - November 21, 2007 at 03:44 PM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
