Do you know the structures of those dyes?: acid blue 40, direct red 23 and disperse yellow 7
Name: Emma
Message: Hi, i am currently in the middle of my A-Level chemistry coursework, and i have done it on the fastness of different dyes on different materials. So far your website has been a fantastic help to not only the theory of my work, but also in doing the practical component too.
I need to know the chemical formula and structure of the 4 dyes i used to complete the write up, so far i have the structure of Procion red MX-5B (found on your site!!) but i still need acid blue 40, direct red 23 and disperse yellow 7 but i am having no luck in finding them.
Do you know the structures of those dyes, they arent on the FAQ section of your site so i hope you can help.
Your first one's very easy. Try a web search with "acid blue 40", including the quotes. The University of South Carolina (as I write this, the top hit in Google) provides both its absorption spectrum and its structure:
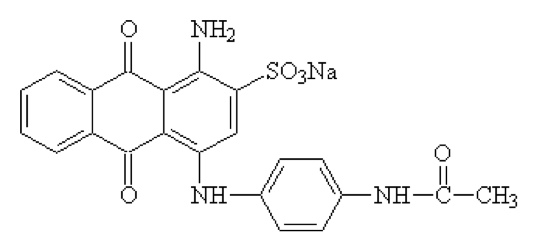
Note the sulfonate group, -SO3Na, important in making the dye soluble in water.
Direct red 23 is a little more difficult. A search for "direct red 23", with the quotes, didn't turn up a page as useful as the one above, although eChinaChem.com gives its molecular formula as C35H25N7Na2O10S2 and its CAS# as 3441-14-3. Searching the catalog of the Sigma Aldritch chemical company yields a drawing of the chemical structure; direct red 23 is a large molecule, as is typical for direct dyes:
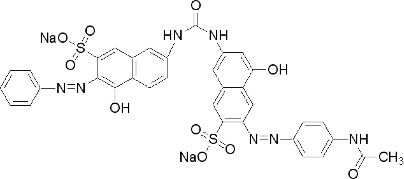
Direct dyes must be large molecules in order for the relatively weak intermolecular interactions to encourage it to stick to cellulose, though direct dyes as a rule are less washfast than acid dyes and much less washfast than fiber reactive dyes. Two sulfonate groups aid in making this dye water soluble. Note that the chemical formula is slightly different than the one given by eChinachem.com.
The online catalog at Sigma Aldritch is also helpful with the structure of disperse yellow 7:
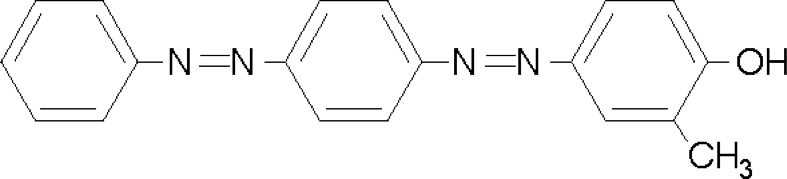
Disperse dyes are typically very poorly water soluble, which is why they are often applied as heat transfers, via dye sublimation, and I think that you can see that this molecule looks pretty much hydrophobic, with only one hydroxyl group and nothing else that looks likely to add water solubility.
(Please help support this web site. Thank you.)
Message: Hi, i am currently in the middle of my A-Level chemistry coursework, and i have done it on the fastness of different dyes on different materials. So far your website has been a fantastic help to not only the theory of my work, but also in doing the practical component too.
I need to know the chemical formula and structure of the 4 dyes i used to complete the write up, so far i have the structure of Procion red MX-5B (found on your site!!) but i still need acid blue 40, direct red 23 and disperse yellow 7 but i am having no luck in finding them.
Do you know the structures of those dyes, they arent on the FAQ section of your site so i hope you can help.
Your first one's very easy. Try a web search with "acid blue 40", including the quotes. The University of South Carolina (as I write this, the top hit in Google) provides both its absorption spectrum and its structure:

Note the sulfonate group, -SO3Na, important in making the dye soluble in water.
Direct red 23 is a little more difficult. A search for "direct red 23", with the quotes, didn't turn up a page as useful as the one above, although eChinaChem.com gives its molecular formula as C35H25N7Na2O10S2 and its CAS# as 3441-14-3. Searching the catalog of the Sigma Aldritch chemical company yields a drawing of the chemical structure; direct red 23 is a large molecule, as is typical for direct dyes:

Direct dyes must be large molecules in order for the relatively weak intermolecular interactions to encourage it to stick to cellulose, though direct dyes as a rule are less washfast than acid dyes and much less washfast than fiber reactive dyes. Two sulfonate groups aid in making this dye water soluble. Note that the chemical formula is slightly different than the one given by eChinachem.com.
The online catalog at Sigma Aldritch is also helpful with the structure of disperse yellow 7:

Disperse dyes are typically very poorly water soluble, which is why they are often applied as heat transfers, via dye sublimation, and I think that you can see that this molecule looks pretty much hydrophobic, with only one hydroxyl group and nothing else that looks likely to add water solubility.
(Please help support this web site. Thank you.)
Posted: Wednesday - March 21, 2007 at 07:42 AM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM