Science fair question: Why did the fabric that was dyed with all ammonia (pH 12) came out lighter than pH 11?
Name: Jodie
Message: I am 12 years old and a middle school student. I recently did a science project titled, What is the Effect of the pH level on the Darkness of the Dyed Fabric?.
I found that the fiber reactive dye, Dylon Cold Water Dye "purple vine", dyed the 100% cotton fabric best at pH 10 and 11 when using a ammonia and water solution to achieve different pH levels. However, at pH 12, the dyed fabric came out very light. For that solution I used 100% Parsons' Ammonia Sudsy Cleaner (composed of ammonium hydroxide solution, anionic surfactant, non-ionic surfactant, opacifier, clarifying agent, and salts [inert] ) and no water. I was wondering if you could help me figure out why the fabric that was dyed with all ammonia (pH 12) came out lighter than pH 11. The temperature for my trials was between 16 and 18 degrees Celsius.
I don't know exactly what dye is in Dylon Cold Water "purple vine", but the manufacturers of Dylon dye say that Dylon Cold Water Dyes contain fiber reactive dyes that are "like" Procion MX dye. I expect that it probably contains Drimarene K dye, which is a type of fiber reactive dye. (If there is any more detailed ingredient information on the package label, please let me know!)
[Added September 26, 2006:
It turns out that Dylon Cold Water Dyes include dichlorotriazine (same as Procion MX) dyes as well as a few Drimarene K and Vinyl Sulfone type dyes. "A19 Purple Vine" includes Colour Index Reactive orange 4 (same as Procion Orange MX-2R), Reactive Red 11 (same as Procion MX red MX-8B), and Reacive Blue 109 (same as Procion MX blue MX-2G); it is possible that it also contains other dyes which are not listed.]
Cotton and most other plant-based fibers are primarily composed of cellulose molecules. Cellulose is a very long molecule formed of a chain of glucose molecules that are attached to each other in a particular way. See, for example, "Cellulose" at London South Bank University.
There are two different possibilities as to why pH 12 was inferior to pH 11. One is simply that the pH optimum, that is, the best pH, for the reaction between dye and fiber is between 10 and 11; trying to do the reaction at too high of a pH is as bad as trying to run it at too low of a pH. Every chemical reaction has a pH at which the reaction proceeds best. Perhaps at a higher pH the dye molecule is more likely to react with water rather than cellulose. For Procion MX dyes, that pH optimum is between 10.2 and 11.0, depending on the specific dye molecule that is used. It appears that Drimarene K has a similar pH optimum, as the recipes advised for home use are identical for these two types of dyes.
The other possible explanation is that ammonium is not the best chemical for adjusting pH upward, and "sudsy" ammonia, unlike "clear" ammonia, contains additional ingredients which may interfere with the reaction; these ingredients would naturally tend to be more of a problem when they are less diluted in water. It would have been better to use clear ammonia, and better still to use soda ash (sodium carbonate) instead. The best temperature for dyeing with this dye is probably 40° C., although as you saw it does work at lower temperatures; see "About Fiber Reactive Dyes".
I do not know why even plain ammonia is never used for dyeing cotton with fiber reactive dyes. Instead, it is usual to use sodium carbonate. The fact that I have never seen a recipe for dyeing cellulose fibers with ammonia, although I have seen mentions of the use of sodium hydroxide or trisodium phosphate, makes me suspect that it is not a good idea. It is sometimes used after dyeing wool. Even if there is nothing wrong with using ammonia instead of sodium carbonate, I would not want to use sudsy ammonia. The soapiness of sudsy ammonia is caused by the surfactants. I certainly would not want to use more than a tiny quantity of any surfactant while dyeing with fiber reactive dyes, but sudsy ammonia contains a large amount of surfactant. I would be afraid that a large amount of surfactant might prevent the dye from reaching the fiber as well. If the dye does not get to the fiber as well, you will get a lighter color.
Fiber reactive dye molecules generally consist of two different sections, one which gives the dye its color, and the other which reacts with the fiber to make a permanent chemical bond. Drimarene K dyes are described as being chlorodifluoropyrimidines. What this means is that the reactive section of the dye is a ring with two fluorine atoms and one chlorine atom sticking out of it. The first step in the dyeing reaction is when a hydroxyl group from the high pH solution reacts with the cellulose in the fiber. One hydrogen atom is removed from an -OH group on the cellulose, creating a negatively charged molecule called a celluosate anion. Next a transient (short-lived) species is formed in which the cellulosate anion attacks the carbon to which one of the halogen atoms is attached; I would guess that the fluorine atom which is closer to the chlorine atom is probably the most likely to be attacked. Finally, the fluorine is lost, leaving a covalently bonded dye-cellulose molecule. This will happen repeatedly along the cellulose fiber. Below (or attached) is a diagram of what I believe to be the most likely reaction mechanism, from information found in John Shore's book Cellulosics Dyeing. You are welcome to use this picture in your project if you reference my web site for it.
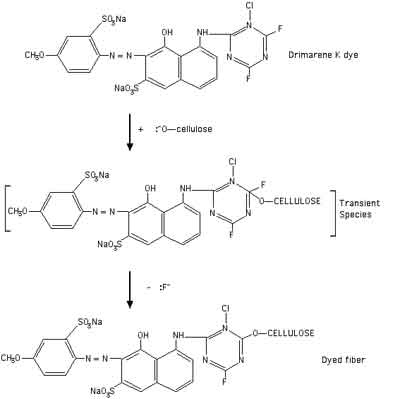
[Note: above image links to larger copy.]
(Please help support this web site. Thank you.)
Message: I am 12 years old and a middle school student. I recently did a science project titled, What is the Effect of the pH level on the Darkness of the Dyed Fabric?.
I found that the fiber reactive dye, Dylon Cold Water Dye "purple vine", dyed the 100% cotton fabric best at pH 10 and 11 when using a ammonia and water solution to achieve different pH levels. However, at pH 12, the dyed fabric came out very light. For that solution I used 100% Parsons' Ammonia Sudsy Cleaner (composed of ammonium hydroxide solution, anionic surfactant, non-ionic surfactant, opacifier, clarifying agent, and salts [inert] ) and no water. I was wondering if you could help me figure out why the fabric that was dyed with all ammonia (pH 12) came out lighter than pH 11. The temperature for my trials was between 16 and 18 degrees Celsius.
I don't know exactly what dye is in Dylon Cold Water "purple vine", but the manufacturers of Dylon dye say that Dylon Cold Water Dyes contain fiber reactive dyes that are "like" Procion MX dye. I expect that it probably contains Drimarene K dye, which is a type of fiber reactive dye. (If there is any more detailed ingredient information on the package label, please let me know!)
[Added September 26, 2006:
It turns out that Dylon Cold Water Dyes include dichlorotriazine (same as Procion MX) dyes as well as a few Drimarene K and Vinyl Sulfone type dyes. "A19 Purple Vine" includes Colour Index Reactive orange 4 (same as Procion Orange MX-2R), Reactive Red 11 (same as Procion MX red MX-8B), and Reacive Blue 109 (same as Procion MX blue MX-2G); it is possible that it also contains other dyes which are not listed.]
Cotton and most other plant-based fibers are primarily composed of cellulose molecules. Cellulose is a very long molecule formed of a chain of glucose molecules that are attached to each other in a particular way. See, for example, "Cellulose" at London South Bank University.
There are two different possibilities as to why pH 12 was inferior to pH 11. One is simply that the pH optimum, that is, the best pH, for the reaction between dye and fiber is between 10 and 11; trying to do the reaction at too high of a pH is as bad as trying to run it at too low of a pH. Every chemical reaction has a pH at which the reaction proceeds best. Perhaps at a higher pH the dye molecule is more likely to react with water rather than cellulose. For Procion MX dyes, that pH optimum is between 10.2 and 11.0, depending on the specific dye molecule that is used. It appears that Drimarene K has a similar pH optimum, as the recipes advised for home use are identical for these two types of dyes.
The other possible explanation is that ammonium is not the best chemical for adjusting pH upward, and "sudsy" ammonia, unlike "clear" ammonia, contains additional ingredients which may interfere with the reaction; these ingredients would naturally tend to be more of a problem when they are less diluted in water. It would have been better to use clear ammonia, and better still to use soda ash (sodium carbonate) instead. The best temperature for dyeing with this dye is probably 40° C., although as you saw it does work at lower temperatures; see "About Fiber Reactive Dyes".
I do not know why even plain ammonia is never used for dyeing cotton with fiber reactive dyes. Instead, it is usual to use sodium carbonate. The fact that I have never seen a recipe for dyeing cellulose fibers with ammonia, although I have seen mentions of the use of sodium hydroxide or trisodium phosphate, makes me suspect that it is not a good idea. It is sometimes used after dyeing wool. Even if there is nothing wrong with using ammonia instead of sodium carbonate, I would not want to use sudsy ammonia. The soapiness of sudsy ammonia is caused by the surfactants. I certainly would not want to use more than a tiny quantity of any surfactant while dyeing with fiber reactive dyes, but sudsy ammonia contains a large amount of surfactant. I would be afraid that a large amount of surfactant might prevent the dye from reaching the fiber as well. If the dye does not get to the fiber as well, you will get a lighter color.
Fiber reactive dye molecules generally consist of two different sections, one which gives the dye its color, and the other which reacts with the fiber to make a permanent chemical bond. Drimarene K dyes are described as being chlorodifluoropyrimidines. What this means is that the reactive section of the dye is a ring with two fluorine atoms and one chlorine atom sticking out of it. The first step in the dyeing reaction is when a hydroxyl group from the high pH solution reacts with the cellulose in the fiber. One hydrogen atom is removed from an -OH group on the cellulose, creating a negatively charged molecule called a celluosate anion. Next a transient (short-lived) species is formed in which the cellulosate anion attacks the carbon to which one of the halogen atoms is attached; I would guess that the fluorine atom which is closer to the chlorine atom is probably the most likely to be attacked. Finally, the fluorine is lost, leaving a covalently bonded dye-cellulose molecule. This will happen repeatedly along the cellulose fiber. Below (or attached) is a diagram of what I believe to be the most likely reaction mechanism, from information found in John Shore's book Cellulosics Dyeing. You are welcome to use this picture in your project if you reference my web site for it.

[Note: above image links to larger copy.]
(Please help support this web site. Thank you.)
Posted: Tuesday - February 21, 2006 at 01:22 PM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM