chemical reaction for a dichlorotriazine dye with cellulose
Name: Catherine
Message: I am a chemistry student doing a project on the chemistry of tie-dying. I understand parts of the reaction, like how the cellulose loses hydrogen atoms and the dye molecules lose the chlorine atoms because of your website. But I am not sure how to formalize it into a chemical equation. Could you possibly give me the equation, using one of the dyes, like the blue MX-R or red MX-8B found on your webpage? If you have time, I would really appreciate it. Thank you,
This is from Chapter 4 in the book Cellulosics Dyeing. The book was edited by John Shore, and the chapter was written by him; see my page of 'Books and Videos on Hand Dyeing and Fabric Painting'....
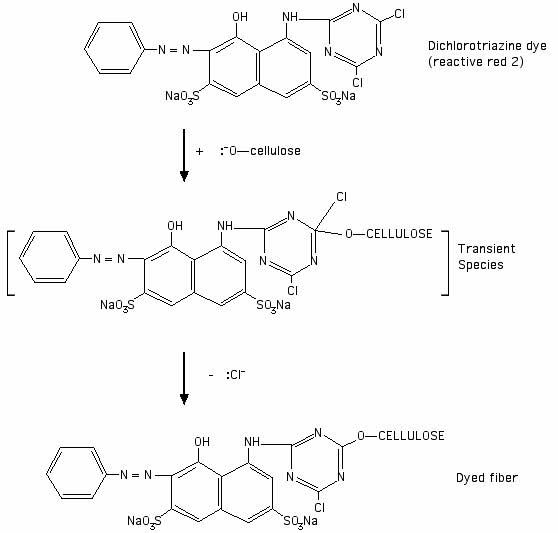
I drew the following illustration of the reaction of reactive red 2 (Procion MX red-5B) with a cellulosate anion, based on an illustration of a generic dichlorotriazine reaction in Cellulosics Dyeing:
Message: I am a chemistry student doing a project on the chemistry of tie-dying. I understand parts of the reaction, like how the cellulose loses hydrogen atoms and the dye molecules lose the chlorine atoms because of your website. But I am not sure how to formalize it into a chemical equation. Could you possibly give me the equation, using one of the dyes, like the blue MX-R or red MX-8B found on your webpage? If you have time, I would really appreciate it. Thank you,
This is from Chapter 4 in the book Cellulosics Dyeing. The book was edited by John Shore, and the chapter was written by him; see my page of 'Books and Videos on Hand Dyeing and Fabric Painting'....
"[Reactive dyes] based on nitrogen-containing heterocyclic rings bearing halogeno substituents undergo nucleophilic substitution. The heteroatoms in the aryl ring activate the system for nucleophilic attack because of their electronegativity. The attacking neutrophile can be either a cellulosate anion or a hydroxide ion, the former leading to fixation on the fibre and the latter resulting in hydrolysis of the reactive dye."
I drew the following illustration of the reaction of reactive red 2 (Procion MX red-5B) with a cellulosate anion, based on an illustration of a generic dichlorotriazine reaction in Cellulosics Dyeing:
Posted: Thursday - May 19, 2005 at 08:56 PM
Follow this blog on twitter here.
Quick Links
- All About Dyes & Dyeing Top -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
- Top of this blog -
- FAQ -
- The Dye Forum -
- How to Tie Dye - How to Batik -
- Books - Toys - Plants -
More in this category:
- -
Statistics
Total entries in this blog:
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM
Total entries in this category:
Published On: Aug 29, 2012 02:48 PM