« 2006 March | Main | 2006 January »
Tuesday, February 28, 2006
I dyed a white cotton jacket black, only it turned out navy. I now want to get it back to white. Is this possible?
Monday, February 27, 2006
Is there an easy or accessible way for me to dye my polyester comforter myself?
Sunday, February 26, 2006
I was hoping to find some fabric for my wedding dress. I'm wanting a gradient from white to pink towards the bottom, and can't seem to find what I want. Do you think it's possible to dye silk so it's a gradiant like that?
Saturday, February 25, 2006
I notice the fabric already printed holds up better, can I put the airbrushed costume in that soda ash I've heard you speak of, in the washer?
Friday, February 24, 2006
I need to dye a white dress shirt to ivory or a light cream color. How would I do this, if it is possible?
Thursday, February 23, 2006
I bought a white sarong mesh, am not sure what kind of fabric it is. I want to dye it and turn it purple.
Wednesday, February 22, 2006
What happens if you use dylon cold dye with salt but not cold fix?
Tuesday, February 21, 2006
Would bleach or the RIT color remover work for just lightening a couple shades?
Science fair question: Why did the fabric that was dyed with all ammonia (pH 12) came out lighter than pH 11?
Monday, February 20, 2006
I'm trying to dye coveralls for my job. I bought a set at Sears, which were blue, and tried to dye them black.
Sunday, February 19, 2006
i am from chinese, i think the tiedyeing and the batik is from chinese, these are many arts is useing this way to get.
Saturday, February 18, 2006
id like to dye some fairy wings, but the material is pretty strange
Friday, February 17, 2006
How practical is it to dye mens underpants from white to black?
Thursday, February 16, 2006
I was wondering if dying it would cover the stain and if so, would I have to dye it a darker colour?
Can I do a "reverse" tie dye on the set of dark purple scrubs I wrecked by splashing bleach?
Can you dye a white satin finish bolero jacket to navy blue with success. I don't know what makes up the satin?
Wednesday, February 15, 2006
I want to fade a new pair of overalls. Can you tell me how much bleach to use in the washer?
Tuesday, February 14, 2006
I noticed a spot on the chest of my sweater that appears lighter in color. If I would dye it black, would this spot cause a problem?
Wednesday, February 08, 2006
What is the best fixative for directly applied deka batik dyes on silk
Tuesday, February 07, 2006
Where can I buy blue Kool-Aid?
Sunday, February 05, 2006
How to tie-dye yarmulkes
Friday, February 03, 2006
I want to remove dye and redye solid embossed velvet. enough for a sofa an two chairs to be recovered.
Thursday, February 02, 2006
5th grade science project on what materials dye the best
I dyed a white cotton jacket black, only it turned out navy. I now want to get it back to white. Is this possible?
Name: Tam
Message: Hello,
I have a 100% cotton jacket (lining is shiny). It was oringinally white and I dyed it black, only it turned out navy. I now want to get it back to white. Is this possible and how do I go about it?
Thanks in anticipation.
Nobody can say without knowing what kind of dye you used. Then again, it is almost always difficult to predict the results of attempting to remove a dye.
If you want to dye cotton black, you should use cool water dyes, not all purpose dye. The best dye to use is a fiber reactive dye such as Procion MX dye or Drimarene K dye (since you're in Australia, see Batik Oetoro), or Tintex Low Temp Dye (not Tintex hot water all-purpose dye), or Dylon Cool Water Dye (not Dylon Multi Purpose). All-purpose dye usually gives disappointing results on cotton. Also, you should use two to four times as much dye as the package instructs, when you are trying to get black.
Removing dye to turn something that is navy to white is unlikely to be 100% successful. Even a tiny bit of remaining dye will make it look dirty. It would make more sense to overdye the jacket to turn it all the way black.
There are two ways to try to remove your dye. One is with the use of ordinary household chlorine bleach (hypochlorite), which will damage any synthetic fiber content on the jacket; it will damage the cotton, too, unless you properly neutralize the bleach afterwards with Anti-Chlor (sodium metabisulfite) or color-safe oxygen 'bleach' such as OxyBoost.
The other is Rit brand Color Remover or Carbona Color Run Remover (sodium hydrosulfite), which is somewhat more gentle to fabric. You will probably need to buy more than one box for a washing machine load, and results cannot be guaranteed. I am not sure if you can find these brand names in Australia where you are, but there should certainly be similar products. Look for Dylon Colour Run Remover or Dylon Run Away to see if they contain sodium hydrosulfite.
(Please help support this web site. Thank you.)
Message: Hello,
I have a 100% cotton jacket (lining is shiny). It was oringinally white and I dyed it black, only it turned out navy. I now want to get it back to white. Is this possible and how do I go about it?
Thanks in anticipation.
Nobody can say without knowing what kind of dye you used. Then again, it is almost always difficult to predict the results of attempting to remove a dye.
If you want to dye cotton black, you should use cool water dyes, not all purpose dye. The best dye to use is a fiber reactive dye such as Procion MX dye or Drimarene K dye (since you're in Australia, see Batik Oetoro), or Tintex Low Temp Dye (not Tintex hot water all-purpose dye), or Dylon Cool Water Dye (not Dylon Multi Purpose). All-purpose dye usually gives disappointing results on cotton. Also, you should use two to four times as much dye as the package instructs, when you are trying to get black.
Removing dye to turn something that is navy to white is unlikely to be 100% successful. Even a tiny bit of remaining dye will make it look dirty. It would make more sense to overdye the jacket to turn it all the way black.
There are two ways to try to remove your dye. One is with the use of ordinary household chlorine bleach (hypochlorite), which will damage any synthetic fiber content on the jacket; it will damage the cotton, too, unless you properly neutralize the bleach afterwards with Anti-Chlor (sodium metabisulfite) or color-safe oxygen 'bleach' such as OxyBoost.
The other is Rit brand Color Remover or Carbona Color Run Remover (sodium hydrosulfite), which is somewhat more gentle to fabric. You will probably need to buy more than one box for a washing machine load, and results cannot be guaranteed. I am not sure if you can find these brand names in Australia where you are, but there should certainly be similar products. Look for Dylon Colour Run Remover or Dylon Run Away to see if they contain sodium hydrosulfite.
(Please help support this web site. Thank you.)
Monday, February 27, 2006
Is there an easy or accessible way for me to dye my polyester comforter myself?
Name: Matt
Message: I have a 100% polyester fill, 100% polyester microfiber cover white comforter that I am wanting to dye black. Is there an easy, or accessible way for me to dye it myself that would not damage, or prevent me from using it? If so, how? Thanks.
The only way to do this is to buy a 100% cotton duvet cover, dye that, and then cover your comforter with it. Or, perhaps you can find a duvet cover which is already the color your want, which would save you quite a bit of effort. Try a web search with 'black duvet cover' and see what you can find.
You cannot dye a comforter that has a polyester cover, because dyeing polyester requires that you somehow find a cooking pot large enough for the item to move in freely as it boils for an hour with special polyester dye. I just can't imagine any way that that could be possible without access to industrial dyeing equipment.
(Please help support this web site. Thank you.)
Message: I have a 100% polyester fill, 100% polyester microfiber cover white comforter that I am wanting to dye black. Is there an easy, or accessible way for me to dye it myself that would not damage, or prevent me from using it? If so, how? Thanks.
The only way to do this is to buy a 100% cotton duvet cover, dye that, and then cover your comforter with it. Or, perhaps you can find a duvet cover which is already the color your want, which would save you quite a bit of effort. Try a web search with 'black duvet cover' and see what you can find.
You cannot dye a comforter that has a polyester cover, because dyeing polyester requires that you somehow find a cooking pot large enough for the item to move in freely as it boils for an hour with special polyester dye. I just can't imagine any way that that could be possible without access to industrial dyeing equipment.
(Please help support this web site. Thank you.)
Sunday, February 26, 2006
I was hoping to find some fabric for my wedding dress. I'm wanting a gradient from white to pink towards the bottom, and can't seem to find what I want. Do you think it's possible to dye silk so it's a gradiant like that?
Name: Gena
Message: Hi!
I was wondering if you maybe have a suggestion for me. I was hoping to find some fabric for my wedding dress. I'm wanting a gradient from white to pink towards the bottom, and can't seem to find what I want. Do you think it's possible to dye silk so it's a gradiant like that? If you've seen Gwen Stefani's wedding gown, it's a good example. Well, thanks for you're time, and I would appreciate any input you may have.
Yes, it is very possible to dye silk that way. What's difficult is when people wish to dye polyester the same way, since polyester is much harder to dye than silk is. Silk is a more beautiful fiber anyway.
I believe that you will probably be able to find a hand dyer listed on my custom dyers list who could do this for you; click on that link to look for a custom dyer. Or look at the professional dyers on my list of links to other galleries to see if there is someone there who does the sort of work you are interested in. It is best to get the fabric yardage dyed before sewing the dress.
One good source for different weaves of silk yardage for dyeing is Silk Connection. (I am not an affiliate of that site.)
(Please help support this web site. Thank you.)
[Link updated November 29, 2007]
Message: Hi!
I was wondering if you maybe have a suggestion for me. I was hoping to find some fabric for my wedding dress. I'm wanting a gradient from white to pink towards the bottom, and can't seem to find what I want. Do you think it's possible to dye silk so it's a gradiant like that? If you've seen Gwen Stefani's wedding gown, it's a good example. Well, thanks for you're time, and I would appreciate any input you may have.
Yes, it is very possible to dye silk that way. What's difficult is when people wish to dye polyester the same way, since polyester is much harder to dye than silk is. Silk is a more beautiful fiber anyway.
I believe that you will probably be able to find a hand dyer listed on my custom dyers list who could do this for you; click on that link to look for a custom dyer. Or look at the professional dyers on my list of links to other galleries to see if there is someone there who does the sort of work you are interested in. It is best to get the fabric yardage dyed before sewing the dress.
One good source for different weaves of silk yardage for dyeing is Silk Connection. (I am not an affiliate of that site.)
(Please help support this web site. Thank you.)
[Link updated November 29, 2007]
Saturday, February 25, 2006
I notice the fabric already printed holds up better, can I put the airbrushed costume in that soda ash I've heard you speak of, in the washer?
Name: penney
Message: Hi, I'm an airbrush artist that does costumes for major theme parks, I have trouble keeping the paint looking good because of the many washes and perspiration of performers, and they are usually synthetic fabrics, I notice the fabric already printed holds up better, can I put the airbrushed costume in that soda ash I've heard you speak of, in the washer? do you think that would work? any help is greatly appreciated
No, sorry, soda ash will not help unless you are using fiber reactive dye. It will do nothing for fabric paints or other types of dye. You can use fiber reactive dyes in an airbrush, but they do not work on synthetics, only on cotton, silk, rayon, and similar fibers. Acid dyes can be used on nylon, but they last best if they are steamed for half an hour or longer, before rinsing.
Heat transfers made with disperse dye should last better on synthetics than airbrushed fabric paint, because the heat drives the vaporized dye a little farther into the fabric. This is the method used for the preprinted synthetic materials. It might be possible for you to prepare similar iron-ons by airbrushing with disperse dye paint onto paper and then using a heat transfer press to transfer to the fabric. We can do the transfer at home with an iron, but the professional heat presses are hotter and larger and therefore give better results. Of course, they cost considerably more. See "Dyeing Polyester with Disperse Dyes" for more general information about disperse dyes, and follow the links to PRO Chemical & Dye to purchase disperse dyes. (I am not affiliated with their site.)
Please be very careful not to inhale any droplets of dye or paint from your airbrushing. I've heard some bad things about airbrush safety lately. Even 'non-toxic' paints can be quite hazardous when inhaled.
(Please help support this web site. Thank you.)
Message: Hi, I'm an airbrush artist that does costumes for major theme parks, I have trouble keeping the paint looking good because of the many washes and perspiration of performers, and they are usually synthetic fabrics, I notice the fabric already printed holds up better, can I put the airbrushed costume in that soda ash I've heard you speak of, in the washer? do you think that would work? any help is greatly appreciated
No, sorry, soda ash will not help unless you are using fiber reactive dye. It will do nothing for fabric paints or other types of dye. You can use fiber reactive dyes in an airbrush, but they do not work on synthetics, only on cotton, silk, rayon, and similar fibers. Acid dyes can be used on nylon, but they last best if they are steamed for half an hour or longer, before rinsing.
Heat transfers made with disperse dye should last better on synthetics than airbrushed fabric paint, because the heat drives the vaporized dye a little farther into the fabric. This is the method used for the preprinted synthetic materials. It might be possible for you to prepare similar iron-ons by airbrushing with disperse dye paint onto paper and then using a heat transfer press to transfer to the fabric. We can do the transfer at home with an iron, but the professional heat presses are hotter and larger and therefore give better results. Of course, they cost considerably more. See "Dyeing Polyester with Disperse Dyes" for more general information about disperse dyes, and follow the links to PRO Chemical & Dye to purchase disperse dyes. (I am not affiliated with their site.)
Please be very careful not to inhale any droplets of dye or paint from your airbrushing. I've heard some bad things about airbrush safety lately. Even 'non-toxic' paints can be quite hazardous when inhaled.
(Please help support this web site. Thank you.)
Friday, February 24, 2006
I need to dye a white dress shirt to ivory or a light cream color. How would I do this, if it is possible?
Name: tammi
Message: I need to dye a white dress shirt to ivory or a light cream color. How would I do this, if it is possible?
What's it made of? Is it 100% cotton?
If you want to dye something a perfect single color, it is best to dye it in the washing machine. Since most washing machines do not allow temperatures above 140°F., you should use a cool water dye, such as Procion MX dye. See "How can I dye clothing or fabric in the washing machine?".
 Dylon Cold Water dyes are fine, too, and include a product called Dylon Tea Dye,
which gives a light-colored result similar to dyeing with real tea, but without
the need to boil the fabric (which is hard on a shirt), and with far more
permanent results.
Dylon Cold Water dyes are fine, too, and include a product called Dylon Tea Dye,
which gives a light-colored result similar to dyeing with real tea, but without
the need to boil the fabric (which is hard on a shirt), and with far more
permanent results.
You could even go ahead and try all-purpose dye with the hottest water your washing machine can provide. All-purpose dyes are usually unsatisfactory on cotton since washing machine temperatures are really too low for them to bond well to cotton, and their colors are duller and tend to wash out a little with every laundering, but for a pale color such as ivory, this should not be much of a problem.
If your shirt is 50% polyester, 50% cotton, you will have to use twice as much dye, since the polyester will not take any of these dyes. Polyester will take a pale brown color if you boil it in coffee, and it is okay to use your good cookware to boil a shirt in coffee, though you should never use it for real dye, but if there is any nylon trim, such as lace or ribbon, it will take a much darker brown color from the coffee than the polyester will.
(Please help support this web site. Thank you.)
Message: I need to dye a white dress shirt to ivory or a light cream color. How would I do this, if it is possible?
What's it made of? Is it 100% cotton?
If you want to dye something a perfect single color, it is best to dye it in the washing machine. Since most washing machines do not allow temperatures above 140°F., you should use a cool water dye, such as Procion MX dye. See "How can I dye clothing or fabric in the washing machine?".
 Dylon Cold Water dyes are fine, too, and include a product called Dylon Tea Dye,
which gives a light-colored result similar to dyeing with real tea, but without
the need to boil the fabric (which is hard on a shirt), and with far more
permanent results.
Dylon Cold Water dyes are fine, too, and include a product called Dylon Tea Dye,
which gives a light-colored result similar to dyeing with real tea, but without
the need to boil the fabric (which is hard on a shirt), and with far more
permanent results. You could even go ahead and try all-purpose dye with the hottest water your washing machine can provide. All-purpose dyes are usually unsatisfactory on cotton since washing machine temperatures are really too low for them to bond well to cotton, and their colors are duller and tend to wash out a little with every laundering, but for a pale color such as ivory, this should not be much of a problem.
If your shirt is 50% polyester, 50% cotton, you will have to use twice as much dye, since the polyester will not take any of these dyes. Polyester will take a pale brown color if you boil it in coffee, and it is okay to use your good cookware to boil a shirt in coffee, though you should never use it for real dye, but if there is any nylon trim, such as lace or ribbon, it will take a much darker brown color from the coffee than the polyester will.
(Please help support this web site. Thank you.)
Thursday, February 23, 2006
I bought a white sarong mesh, am not sure what kind of fabric it is. I want to dye it and turn it purple.
Name: michelle
Message: i bought a white sarong mesh, am not sure what kind of fabric it is.. i want to dye it and turn it into purple.. what should i do to make it chlorine resistant since i'll be using it for a pool party.. thanks.. i hope you can help me...
I can't really tell you without knowing what kind of fabric it is. The fiber content should be clearly marked when you purchase a garment.
If it's polyester, you probably don't want to dye it. You'd have to get a large non-aluminum pot to use for dyeing, and never use it for food again - rather a large investment for an occasional dyer. Also, you cannot dye polyester/spandex because spandex is easily damaged by heat, whereas polyester requires heat to dye.
The best sarongs for dyeing are made of cotton, rayon, or silk - all 100% natural fibers. PFD (prepared for dyeing) silk, cotton, or rayon sarongs cost from $6 to $10 at Silk Connection or Dharma Trading Company (see contact info on my Sources for Supplies links page). You can dye them with cool water Procion MX dyes, which are fairly resistant to chlorine. I would recommend against the use of all-purpose dye on these fibers, because it often produces dull colored results, and it bleeds a tiny bit every time it gets wet, so it does not last.
(Please help support this web site. Thank you.)
Message: i bought a white sarong mesh, am not sure what kind of fabric it is.. i want to dye it and turn it into purple.. what should i do to make it chlorine resistant since i'll be using it for a pool party.. thanks.. i hope you can help me...
I can't really tell you without knowing what kind of fabric it is. The fiber content should be clearly marked when you purchase a garment.
If it's polyester, you probably don't want to dye it. You'd have to get a large non-aluminum pot to use for dyeing, and never use it for food again - rather a large investment for an occasional dyer. Also, you cannot dye polyester/spandex because spandex is easily damaged by heat, whereas polyester requires heat to dye.
The best sarongs for dyeing are made of cotton, rayon, or silk - all 100% natural fibers. PFD (prepared for dyeing) silk, cotton, or rayon sarongs cost from $6 to $10 at Silk Connection or Dharma Trading Company (see contact info on my Sources for Supplies links page). You can dye them with cool water Procion MX dyes, which are fairly resistant to chlorine. I would recommend against the use of all-purpose dye on these fibers, because it often produces dull colored results, and it bleeds a tiny bit every time it gets wet, so it does not last.
(Please help support this web site. Thank you.)
Wednesday, February 22, 2006
What happens if you use dylon cold dye with salt but not cold fix?
Name: gillian
Message: what happens if you use dylon cold dye with salt but not cold fix what happens
It just washes out. Fiber reactive dyes, such as Dylon Cold Water Dye, require a high pH to fix to the fabric. Salt does not set dye.
You don't have to use Dylon brand Cold Fix, however. You can use sodium carbonate, or soda ash, which is sold among swimming pool supplies to raise the pH of pool water.
You may substitute three times as much washing soda, if you can find a brand that is free of whiteners and brighteners.
(Please help support this web site. Thank you.)
Message: what happens if you use dylon cold dye with salt but not cold fix what happens
It just washes out. Fiber reactive dyes, such as Dylon Cold Water Dye, require a high pH to fix to the fabric. Salt does not set dye.
You don't have to use Dylon brand Cold Fix, however. You can use sodium carbonate, or soda ash, which is sold among swimming pool supplies to raise the pH of pool water.
You may substitute three times as much washing soda, if you can find a brand that is free of whiteners and brighteners.
(Please help support this web site. Thank you.)
Tuesday, February 21, 2006
Would bleach or the RIT color remover work for just lightening a couple shades?
Name: Savannah
Message: I have a pair of 100% opaque cotton tights, and they're a lovely green color. My issue is that I need them a few shades lighter for them to be accurate to the costume I'm going for. Would bleach or the RIT color remover work for just lightening a couple shades? A recommendation would be much appreciated. :)
I think the Color Remover would be safer, especially if there is ANY spandex in the tights to make them stretchy. Chlorine bleach is so bad for spandex or nylon.
 I have used Rit Color Remover to lighten something a few shades. I used one box
for a washing machine load that should have required more than one, and I
hovered over the washing machine and set it to drain when the right color was
reached. You'd have more control if you used a large bucket or the bathtub,
stirring frequently, and then plunged them immediately into clean water, and
then launder, as soon as the desired color is reached - keeping in mind that
everything looks darker when it is still wet than it will when it is
dry.
I have used Rit Color Remover to lighten something a few shades. I used one box
for a washing machine load that should have required more than one, and I
hovered over the washing machine and set it to drain when the right color was
reached. You'd have more control if you used a large bucket or the bathtub,
stirring frequently, and then plunged them immediately into clean water, and
then launder, as soon as the desired color is reached - keeping in mind that
everything looks darker when it is still wet than it will when it is
dry.
It is not possible to predict how well Color Remover or bleach will work to remove the dye. Some dye colors discharge nicely, some change color, and some are unchanged. Good luck.
(Please help support this web site. Thank you.)
Message: I have a pair of 100% opaque cotton tights, and they're a lovely green color. My issue is that I need them a few shades lighter for them to be accurate to the costume I'm going for. Would bleach or the RIT color remover work for just lightening a couple shades? A recommendation would be much appreciated. :)
I think the Color Remover would be safer, especially if there is ANY spandex in the tights to make them stretchy. Chlorine bleach is so bad for spandex or nylon.
 I have used Rit Color Remover to lighten something a few shades. I used one box
for a washing machine load that should have required more than one, and I
hovered over the washing machine and set it to drain when the right color was
reached. You'd have more control if you used a large bucket or the bathtub,
stirring frequently, and then plunged them immediately into clean water, and
then launder, as soon as the desired color is reached - keeping in mind that
everything looks darker when it is still wet than it will when it is
dry.
I have used Rit Color Remover to lighten something a few shades. I used one box
for a washing machine load that should have required more than one, and I
hovered over the washing machine and set it to drain when the right color was
reached. You'd have more control if you used a large bucket or the bathtub,
stirring frequently, and then plunged them immediately into clean water, and
then launder, as soon as the desired color is reached - keeping in mind that
everything looks darker when it is still wet than it will when it is
dry.It is not possible to predict how well Color Remover or bleach will work to remove the dye. Some dye colors discharge nicely, some change color, and some are unchanged. Good luck.
(Please help support this web site. Thank you.)
Science fair question: Why did the fabric that was dyed with all ammonia (pH 12) came out lighter than pH 11?
Name: Jodie
Message: I am 12 years old and a middle school student. I recently did a science project titled, What is the Effect of the pH level on the Darkness of the Dyed Fabric?.
I found that the fiber reactive dye, Dylon Cold Water Dye "purple vine", dyed the 100% cotton fabric best at pH 10 and 11 when using a ammonia and water solution to achieve different pH levels. However, at pH 12, the dyed fabric came out very light. For that solution I used 100% Parsons' Ammonia Sudsy Cleaner (composed of ammonium hydroxide solution, anionic surfactant, non-ionic surfactant, opacifier, clarifying agent, and salts [inert] ) and no water. I was wondering if you could help me figure out why the fabric that was dyed with all ammonia (pH 12) came out lighter than pH 11. The temperature for my trials was between 16 and 18 degrees Celsius.
I don't know exactly what dye is in Dylon Cold Water "purple vine", but the manufacturers of Dylon dye say that Dylon Cold Water Dyes contain fiber reactive dyes that are "like" Procion MX dye. I expect that it probably contains Drimarene K dye, which is a type of fiber reactive dye. (If there is any more detailed ingredient information on the package label, please let me know!)
[Added September 26, 2006:
It turns out that Dylon Cold Water Dyes include dichlorotriazine (same as Procion MX) dyes as well as a few Drimarene K and Vinyl Sulfone type dyes. "A19 Purple Vine" includes Colour Index Reactive orange 4 (same as Procion Orange MX-2R), Reactive Red 11 (same as Procion MX red MX-8B), and Reacive Blue 109 (same as Procion MX blue MX-2G); it is possible that it also contains other dyes which are not listed.]
Cotton and most other plant-based fibers are primarily composed of cellulose molecules. Cellulose is a very long molecule formed of a chain of glucose molecules that are attached to each other in a particular way. See, for example, "Cellulose" at London South Bank University.
There are two different possibilities as to why pH 12 was inferior to pH 11. One is simply that the pH optimum, that is, the best pH, for the reaction between dye and fiber is between 10 and 11; trying to do the reaction at too high of a pH is as bad as trying to run it at too low of a pH. Every chemical reaction has a pH at which the reaction proceeds best. Perhaps at a higher pH the dye molecule is more likely to react with water rather than cellulose. For Procion MX dyes, that pH optimum is between 10.2 and 11.0, depending on the specific dye molecule that is used. It appears that Drimarene K has a similar pH optimum, as the recipes advised for home use are identical for these two types of dyes.
The other possible explanation is that ammonium is not the best chemical for adjusting pH upward, and "sudsy" ammonia, unlike "clear" ammonia, contains additional ingredients which may interfere with the reaction; these ingredients would naturally tend to be more of a problem when they are less diluted in water. It would have been better to use clear ammonia, and better still to use soda ash (sodium carbonate) instead. The best temperature for dyeing with this dye is probably 40° C., although as you saw it does work at lower temperatures; see "About Fiber Reactive Dyes".
I do not know why even plain ammonia is never used for dyeing cotton with fiber reactive dyes. Instead, it is usual to use sodium carbonate. The fact that I have never seen a recipe for dyeing cellulose fibers with ammonia, although I have seen mentions of the use of sodium hydroxide or trisodium phosphate, makes me suspect that it is not a good idea. It is sometimes used after dyeing wool. Even if there is nothing wrong with using ammonia instead of sodium carbonate, I would not want to use sudsy ammonia. The soapiness of sudsy ammonia is caused by the surfactants. I certainly would not want to use more than a tiny quantity of any surfactant while dyeing with fiber reactive dyes, but sudsy ammonia contains a large amount of surfactant. I would be afraid that a large amount of surfactant might prevent the dye from reaching the fiber as well. If the dye does not get to the fiber as well, you will get a lighter color.
Fiber reactive dye molecules generally consist of two different sections, one which gives the dye its color, and the other which reacts with the fiber to make a permanent chemical bond. Drimarene K dyes are described as being chlorodifluoropyrimidines. What this means is that the reactive section of the dye is a ring with two fluorine atoms and one chlorine atom sticking out of it. The first step in the dyeing reaction is when a hydroxyl group from the high pH solution reacts with the cellulose in the fiber. One hydrogen atom is removed from an -OH group on the cellulose, creating a negatively charged molecule called a celluosate anion. Next a transient (short-lived) species is formed in which the cellulosate anion attacks the carbon to which one of the halogen atoms is attached; I would guess that the fluorine atom which is closer to the chlorine atom is probably the most likely to be attacked. Finally, the fluorine is lost, leaving a covalently bonded dye-cellulose molecule. This will happen repeatedly along the cellulose fiber. Below (or attached) is a diagram of what I believe to be the most likely reaction mechanism, from information found in John Shore's book Cellulosics Dyeing. You are welcome to use this picture in your project if you reference my web site for it.
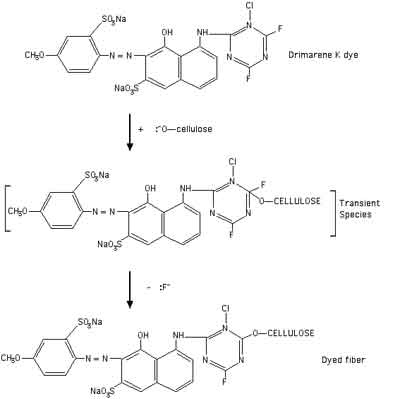
[Note: above image links to larger copy.]
(Please help support this web site. Thank you.)
Message: I am 12 years old and a middle school student. I recently did a science project titled, What is the Effect of the pH level on the Darkness of the Dyed Fabric?.
I found that the fiber reactive dye, Dylon Cold Water Dye "purple vine", dyed the 100% cotton fabric best at pH 10 and 11 when using a ammonia and water solution to achieve different pH levels. However, at pH 12, the dyed fabric came out very light. For that solution I used 100% Parsons' Ammonia Sudsy Cleaner (composed of ammonium hydroxide solution, anionic surfactant, non-ionic surfactant, opacifier, clarifying agent, and salts [inert] ) and no water. I was wondering if you could help me figure out why the fabric that was dyed with all ammonia (pH 12) came out lighter than pH 11. The temperature for my trials was between 16 and 18 degrees Celsius.
I don't know exactly what dye is in Dylon Cold Water "purple vine", but the manufacturers of Dylon dye say that Dylon Cold Water Dyes contain fiber reactive dyes that are "like" Procion MX dye. I expect that it probably contains Drimarene K dye, which is a type of fiber reactive dye. (If there is any more detailed ingredient information on the package label, please let me know!)
[Added September 26, 2006:
It turns out that Dylon Cold Water Dyes include dichlorotriazine (same as Procion MX) dyes as well as a few Drimarene K and Vinyl Sulfone type dyes. "A19 Purple Vine" includes Colour Index Reactive orange 4 (same as Procion Orange MX-2R), Reactive Red 11 (same as Procion MX red MX-8B), and Reacive Blue 109 (same as Procion MX blue MX-2G); it is possible that it also contains other dyes which are not listed.]
Cotton and most other plant-based fibers are primarily composed of cellulose molecules. Cellulose is a very long molecule formed of a chain of glucose molecules that are attached to each other in a particular way. See, for example, "Cellulose" at London South Bank University.
There are two different possibilities as to why pH 12 was inferior to pH 11. One is simply that the pH optimum, that is, the best pH, for the reaction between dye and fiber is between 10 and 11; trying to do the reaction at too high of a pH is as bad as trying to run it at too low of a pH. Every chemical reaction has a pH at which the reaction proceeds best. Perhaps at a higher pH the dye molecule is more likely to react with water rather than cellulose. For Procion MX dyes, that pH optimum is between 10.2 and 11.0, depending on the specific dye molecule that is used. It appears that Drimarene K has a similar pH optimum, as the recipes advised for home use are identical for these two types of dyes.
The other possible explanation is that ammonium is not the best chemical for adjusting pH upward, and "sudsy" ammonia, unlike "clear" ammonia, contains additional ingredients which may interfere with the reaction; these ingredients would naturally tend to be more of a problem when they are less diluted in water. It would have been better to use clear ammonia, and better still to use soda ash (sodium carbonate) instead. The best temperature for dyeing with this dye is probably 40° C., although as you saw it does work at lower temperatures; see "About Fiber Reactive Dyes".
I do not know why even plain ammonia is never used for dyeing cotton with fiber reactive dyes. Instead, it is usual to use sodium carbonate. The fact that I have never seen a recipe for dyeing cellulose fibers with ammonia, although I have seen mentions of the use of sodium hydroxide or trisodium phosphate, makes me suspect that it is not a good idea. It is sometimes used after dyeing wool. Even if there is nothing wrong with using ammonia instead of sodium carbonate, I would not want to use sudsy ammonia. The soapiness of sudsy ammonia is caused by the surfactants. I certainly would not want to use more than a tiny quantity of any surfactant while dyeing with fiber reactive dyes, but sudsy ammonia contains a large amount of surfactant. I would be afraid that a large amount of surfactant might prevent the dye from reaching the fiber as well. If the dye does not get to the fiber as well, you will get a lighter color.
Fiber reactive dye molecules generally consist of two different sections, one which gives the dye its color, and the other which reacts with the fiber to make a permanent chemical bond. Drimarene K dyes are described as being chlorodifluoropyrimidines. What this means is that the reactive section of the dye is a ring with two fluorine atoms and one chlorine atom sticking out of it. The first step in the dyeing reaction is when a hydroxyl group from the high pH solution reacts with the cellulose in the fiber. One hydrogen atom is removed from an -OH group on the cellulose, creating a negatively charged molecule called a celluosate anion. Next a transient (short-lived) species is formed in which the cellulosate anion attacks the carbon to which one of the halogen atoms is attached; I would guess that the fluorine atom which is closer to the chlorine atom is probably the most likely to be attacked. Finally, the fluorine is lost, leaving a covalently bonded dye-cellulose molecule. This will happen repeatedly along the cellulose fiber. Below (or attached) is a diagram of what I believe to be the most likely reaction mechanism, from information found in John Shore's book Cellulosics Dyeing. You are welcome to use this picture in your project if you reference my web site for it.

[Note: above image links to larger copy.]
(Please help support this web site. Thank you.)
Monday, February 20, 2006
I'm trying to dye coveralls for my job. I bought a set at Sears, which were blue, and tried to dye them black.
Name: Barry
Message: I'm trying to dye coveralls for my job. I bought a set at Sears, which were blue, and tried to dye them black. I thought bleaching them first would help, but after dying them, the coveralls are a dark brown. I'm using RIT, salt and my washer with hot water. I need black to work because I'm in printing and constantly around black toner and developer. I bought 2 more pairs of coveralls both white this time. They are 65/35 polyester-cotton. Can it work? I read that polyester may not dye wellfrom this website, but maybe you have a trick up your sleeve? And don't say "Buy Black Coveralls", nobody sells them !!! Unbelievable, right? Thank you.
All-purpose dye does not yield the best results on cotton, and does not work at all on polyester. I cannot recommend its use, except for dyeing wool and nylon. It will bleed in the laundry forever, and will not stay dark for long. You will be much happier using cool water fiber reactive dye, but unfortunately this type of dye does not work on polyester, only on cotton and other natural fibers.
65% polyester is difficult to dye. It is possible to dye polyester by boiling it with disperse dye, which you can buy by mail-order from PRO Chemical & Dye. Unfortunately, you cannot use disperse dye without extremely high heat. It is used either by boiling with a carrier chemical, or by ironing on after first painting or coloring it on to paper. It is difficult to get a solid color effect with the iron-on technique, however.
If what you want is black, there's no need to bleach out the existing color. To dye black, it is best to start with as dark a color as you can get. Bleaching tends to damage polyester badly, turning it permanently yellow and possibly changing its hand (the way it feels to the touch).
Fabric paint can be used to 'pigment dye' polyester. However, I am not sure how dark you will be able to get it. For example, see the description of Dharma Trading Company's pigment 'dyes'.
Perhaps this web site is new since you did your searching: http://www.coverallsale.com. Apparently they sell several different styles of cotton coveralls (and they might actually have black poly/cotton overalls!) There may be other possible sources, as well. Cotton coveralls would be very easy to dye with ordinary fiber reactive dyes. The thread will stay the original color, but cotton fabric is easy to dye, if it is not treated with a permanent press or stain resistant finish. I recommend that you purchase Procion MX dye from any of the dye suppliers around the world listed on my Sources for Dyeing Supplies page. Any sort of fiber reactive dye would be suitable, including Dylon Cold Water Dye.
One last point: when dyeing black, use two to four times as much dye as the package suggests. Black requires more dye than other colors do.
(Please help support this web site. Thank you.)
Message: I'm trying to dye coveralls for my job. I bought a set at Sears, which were blue, and tried to dye them black. I thought bleaching them first would help, but after dying them, the coveralls are a dark brown. I'm using RIT, salt and my washer with hot water. I need black to work because I'm in printing and constantly around black toner and developer. I bought 2 more pairs of coveralls both white this time. They are 65/35 polyester-cotton. Can it work? I read that polyester may not dye wellfrom this website, but maybe you have a trick up your sleeve? And don't say "Buy Black Coveralls", nobody sells them !!! Unbelievable, right? Thank you.
All-purpose dye does not yield the best results on cotton, and does not work at all on polyester. I cannot recommend its use, except for dyeing wool and nylon. It will bleed in the laundry forever, and will not stay dark for long. You will be much happier using cool water fiber reactive dye, but unfortunately this type of dye does not work on polyester, only on cotton and other natural fibers.
65% polyester is difficult to dye. It is possible to dye polyester by boiling it with disperse dye, which you can buy by mail-order from PRO Chemical & Dye. Unfortunately, you cannot use disperse dye without extremely high heat. It is used either by boiling with a carrier chemical, or by ironing on after first painting or coloring it on to paper. It is difficult to get a solid color effect with the iron-on technique, however.
If what you want is black, there's no need to bleach out the existing color. To dye black, it is best to start with as dark a color as you can get. Bleaching tends to damage polyester badly, turning it permanently yellow and possibly changing its hand (the way it feels to the touch).
Fabric paint can be used to 'pigment dye' polyester. However, I am not sure how dark you will be able to get it. For example, see the description of Dharma Trading Company's pigment 'dyes'.
Perhaps this web site is new since you did your searching: http://www.coverallsale.com. Apparently they sell several different styles of cotton coveralls (and they might actually have black poly/cotton overalls!) There may be other possible sources, as well. Cotton coveralls would be very easy to dye with ordinary fiber reactive dyes. The thread will stay the original color, but cotton fabric is easy to dye, if it is not treated with a permanent press or stain resistant finish. I recommend that you purchase Procion MX dye from any of the dye suppliers around the world listed on my Sources for Dyeing Supplies page. Any sort of fiber reactive dye would be suitable, including Dylon Cold Water Dye.
One last point: when dyeing black, use two to four times as much dye as the package suggests. Black requires more dye than other colors do.
(Please help support this web site. Thank you.)
Sunday, February 19, 2006
i am from chinese, i think the tiedyeing and the batik is from chinese, these are many arts is useing this way to get.
Name: chinaashima
Message: i am from chinese, i think the tiedyeing and the batik is from chinese, these are many arts is useing this way to get. i want to intercommunion about these.
Yes, it is true that one of the earliest known examples of resist dyeing (tie dyeing or batik) was found in a Chinese tomb from 1600 years ago. Tie-dyeing was also developed independently in other countries, such as Peru and India, and different resist techniques may also have been developed more than once in different cultures.
An interesting web page to look at for more information on the history of resist dyeing may be found at the World Shibori Network.
My own page on the history of tie-dyeing is "A Few Notes on the History of Tie Dye".
(Please help support this web site. Thank you.)
Message: i am from chinese, i think the tiedyeing and the batik is from chinese, these are many arts is useing this way to get. i want to intercommunion about these.
Yes, it is true that one of the earliest known examples of resist dyeing (tie dyeing or batik) was found in a Chinese tomb from 1600 years ago. Tie-dyeing was also developed independently in other countries, such as Peru and India, and different resist techniques may also have been developed more than once in different cultures.
An interesting web page to look at for more information on the history of resist dyeing may be found at the World Shibori Network.
My own page on the history of tie-dyeing is "A Few Notes on the History of Tie Dye".
(Please help support this web site. Thank you.)
Saturday, February 18, 2006
id like to dye some fairy wings, but the material is pretty strange
Name: ruthie
Message: Hi,
id like to dye some fairy wings, but the material is pretty strange, you know like a stretchy netting>? Almost like tights. Also its a pretty awkward shape and I live in rented accomodation so dont really wanna ruin the bathtub!
Any ideas as what to use?
Thanks so much
ps, what a great site, lots of info!
The problem is deciding what type of dye to use. You have to know what kind of fabric you have in order to choose the dye. Is it made of nylon?
If what you have is nylon/lycra, you can dye it with acid dyes. The most readily available acid dyes are found in the mixture of dyes in all-purpose dye, such as Rit brand dye. All-purpose dye is not very good on cotton but should work on nylon.
Acid dye will work best in water that is much hotter than tap water, but it is possible to use it in a washing machine; see the instructions on "How can I dye clothing or fabric in the washing machine?". You should not use a cooking pot for dyeing unless you are never going to use it for food again - unless you try dying with food coloring, which is another type of acid dye. See "How can I tie dye with Kool-aid?". Note that while food coloring can be used on nylon or wool, it will not work at all as dye on cotton.
Synthetics other than nylon will be very difficult to dye. Acid dye will just rinse out of polyester or acetate. The only dye that works on polyester, disperse dye, requires high heat to transfer, but lycra, which is the stretchy stuff, will not tolerate hight heat.
If your stretchy material is made of polyester and lycra, your only hope is to use fabric paint, instead of dye.
(Please help support this web site. Thank you.)
Message: Hi,
id like to dye some fairy wings, but the material is pretty strange, you know like a stretchy netting>? Almost like tights. Also its a pretty awkward shape and I live in rented accomodation so dont really wanna ruin the bathtub!
Any ideas as what to use?
Thanks so much
ps, what a great site, lots of info!
The problem is deciding what type of dye to use. You have to know what kind of fabric you have in order to choose the dye. Is it made of nylon?
If what you have is nylon/lycra, you can dye it with acid dyes. The most readily available acid dyes are found in the mixture of dyes in all-purpose dye, such as Rit brand dye. All-purpose dye is not very good on cotton but should work on nylon.
Acid dye will work best in water that is much hotter than tap water, but it is possible to use it in a washing machine; see the instructions on "How can I dye clothing or fabric in the washing machine?". You should not use a cooking pot for dyeing unless you are never going to use it for food again - unless you try dying with food coloring, which is another type of acid dye. See "How can I tie dye with Kool-aid?". Note that while food coloring can be used on nylon or wool, it will not work at all as dye on cotton.
Synthetics other than nylon will be very difficult to dye. Acid dye will just rinse out of polyester or acetate. The only dye that works on polyester, disperse dye, requires high heat to transfer, but lycra, which is the stretchy stuff, will not tolerate hight heat.
If your stretchy material is made of polyester and lycra, your only hope is to use fabric paint, instead of dye.
(Please help support this web site. Thank you.)
Friday, February 17, 2006
How practical is it to dye mens underpants from white to black?
Name: Larry
Message: How practical is it to dye mens underpants from white to black? My GF doesn't like the "tightywhiteys" I wear but colored Jockeys cost so much more than the whites. Besides I hate to throw away usable clothing.
Very practical indeed! I've dyed 100% cotton underwear many times. Of course I usually dye them different bright colors, using tie-dye or preferably low water immersion dyeing, rather than black.
The most important thing to do is choose the right dye. All-purpose dye is easy to find in stores, but tends to produce grey rather than black, and bleeds onto everything else in the laundry for the life of the garment. Not very practical at all! It's a reasonable choice for nylon underwear, however, and especially for cotton underwear that is covered with nylon lace.
Fiber reactive dye, such as Procion MX dye, gives much better results. Buy it by mail-order from any of the dye supply companies around the world listed on my Sources for Supplies page. If you want solid colors, follow the instructions found on "How can I dye clothing or fabric in the washing machine?". If you want something a little more complex, but not tie-dyed, consider low water immersion; combining a black dye mixture with blue or green would produce nice results. See "How to Do Low Water Immersion Dyeing".
One problem you will find in dyeing readymade white garments is that the polyester thread that was used to sew them together will stay white. If the stitching is even, however, this will look intentional, like decorative top-stitching. I do not know of any source for underwear sewn with dyeable cotton thread.
(Please help support this web site. Thank you.)
Message: How practical is it to dye mens underpants from white to black? My GF doesn't like the "tightywhiteys" I wear but colored Jockeys cost so much more than the whites. Besides I hate to throw away usable clothing.
Very practical indeed! I've dyed 100% cotton underwear many times. Of course I usually dye them different bright colors, using tie-dye or preferably low water immersion dyeing, rather than black.
The most important thing to do is choose the right dye. All-purpose dye is easy to find in stores, but tends to produce grey rather than black, and bleeds onto everything else in the laundry for the life of the garment. Not very practical at all! It's a reasonable choice for nylon underwear, however, and especially for cotton underwear that is covered with nylon lace.
Fiber reactive dye, such as Procion MX dye, gives much better results. Buy it by mail-order from any of the dye supply companies around the world listed on my Sources for Supplies page. If you want solid colors, follow the instructions found on "How can I dye clothing or fabric in the washing machine?". If you want something a little more complex, but not tie-dyed, consider low water immersion; combining a black dye mixture with blue or green would produce nice results. See "How to Do Low Water Immersion Dyeing".
One problem you will find in dyeing readymade white garments is that the polyester thread that was used to sew them together will stay white. If the stitching is even, however, this will look intentional, like decorative top-stitching. I do not know of any source for underwear sewn with dyeable cotton thread.
(Please help support this web site. Thank you.)
Thursday, February 16, 2006
I was wondering if dying it would cover the stain and if so, would I have to dye it a darker colour?
Name: Stefanie
Message: I have a silk top that I got blood on. I have sent it to the dry cleaners to try to get it out, but no luck. I was wondering if dying it would cover the stain and if so, would I have to dye it a darker colour? It's a beautiful jewel-tone blue
I'm sorry, but it is difficult to cover a stain by dyeing, because the darker stain shows through the dye, which is transparent, and remains darker than the rest of the garment. Only a very dark color has any real hope of covering up the stain.
Your shirt is probably ruined. The best way to remove a bloodstain is to soak it immediately in cold water, then wash it in cool water. If this does not work, sometimes you can bleach out the stain with hydrogen peroxide, purchased as an antiseptic from a pharmacy, though there is always a risk that the original dye in the shirt will be damaged as well.
(Please help support this web site. Thank you.)
Message: I have a silk top that I got blood on. I have sent it to the dry cleaners to try to get it out, but no luck. I was wondering if dying it would cover the stain and if so, would I have to dye it a darker colour? It's a beautiful jewel-tone blue
I'm sorry, but it is difficult to cover a stain by dyeing, because the darker stain shows through the dye, which is transparent, and remains darker than the rest of the garment. Only a very dark color has any real hope of covering up the stain.
Your shirt is probably ruined. The best way to remove a bloodstain is to soak it immediately in cold water, then wash it in cool water. If this does not work, sometimes you can bleach out the stain with hydrogen peroxide, purchased as an antiseptic from a pharmacy, though there is always a risk that the original dye in the shirt will be damaged as well.
(Please help support this web site. Thank you.)
Can I do a "reverse" tie dye on the set of dark purple scrubs I wrecked by splashing bleach?
Name: Kendall
Message: I am a surgical nurse who has wrecked a set of dark purple scrubs by splashing bleach. Of course the areas hit by the bleach make me look like I am breast feeding and have bladder control issues (smirk) Can I do a "reverse" tie die and look like a groovy nurse instead of a leaking nurse?
Sure! See "How to Tie Dye on Dark Fabric". Also see "How can I fix the bleach spots on my favorite clothing?".
You will need to neutralize the bleach, to extend the life of the garments, as is detailed on the above pages.
Another nice effect is to bleach out some areas and then overdye. Use fiber reactive dye, not all-purpose dye, if you want to dye cotton.
(Please help support this web site. Thank you.)
Message: I am a surgical nurse who has wrecked a set of dark purple scrubs by splashing bleach. Of course the areas hit by the bleach make me look like I am breast feeding and have bladder control issues (smirk) Can I do a "reverse" tie die and look like a groovy nurse instead of a leaking nurse?
Sure! See "How to Tie Dye on Dark Fabric". Also see "How can I fix the bleach spots on my favorite clothing?".
You will need to neutralize the bleach, to extend the life of the garments, as is detailed on the above pages.
Another nice effect is to bleach out some areas and then overdye. Use fiber reactive dye, not all-purpose dye, if you want to dye cotton.
(Please help support this web site. Thank you.)
Can you dye a white satin finish bolero jacket to navy blue with success. I don't know what makes up the satin?
Name: Bobbie
Message: Can you dye a white satin finish bolero jacket to navy blue with success. I don't know what makes up the satin?
No, if you don't know what the fiber is, you don't know what kind of dye to use. You have to match your dye to the fiber content. Satin is a weave that can be made with almost any fiber, natural or synthetic.
Silk satin is very easy to dye, using either fiber reactive dyes, such as Procion MX dyes, or acid dyes. Polyester satin is very difficult to dye; the only dye that will work is disperse dye, which must be boiled with the jacket for an hour. I suspect that your jacket might not appreciate being boiled.
(Please help support this web site. Thank you.)
Message: Can you dye a white satin finish bolero jacket to navy blue with success. I don't know what makes up the satin?
No, if you don't know what the fiber is, you don't know what kind of dye to use. You have to match your dye to the fiber content. Satin is a weave that can be made with almost any fiber, natural or synthetic.
Silk satin is very easy to dye, using either fiber reactive dyes, such as Procion MX dyes, or acid dyes. Polyester satin is very difficult to dye; the only dye that will work is disperse dye, which must be boiled with the jacket for an hour. I suspect that your jacket might not appreciate being boiled.
(Please help support this web site. Thank you.)
Wednesday, February 15, 2006
I want to fade a new pair of overalls. Can you tell me how much bleach to use in the washer?
Name: Gail
Message: I want to fade a new pair of extra large overalls. Can you tell me how much bleach to use in the washer? Should I add the bleach to the wait and let it agitate until mixed well before I add the overalls? I don't want them to be spotted.
Whatever discharge agent you use to lighten your overalls, you should certainly mix it well with the water in the washing machine before adding them. Or, you can mix it into a quart of water and add it to the automatic bleach dispenser on your washing machine, if there is one.
You have two main choices for chemicals to use to lighten the dye. The best choice would probably be Rit Fast Fade for Jeans, which is often available from fabric stores. Rit Fast Fade for Jeans contains sodium carbonate and sodium dichloroisocyanurate dihydrate. It is easier on the fabric than chlorine bleach, and is less apt to leave holes. Follow the instructions on the box.
The other choice is chlorine bleach, which contains sodium hypochlorite. Chlorine bleach will continue to eat away at the denim after you have finished using it, unless you neutralize it afterwards. You can neutralize it with a product called Anti-Chlor (sodium metabisulfite), or by rinsing with hydrogen peroxide or by washing with a chlorine-free oxygen 'bleach', such as OxyBoost. Do not attempt to use vinegar for this purpose, as the reaction between bleach and acid produces even more caustic and dangerous chemicals. I am not sure how much chlorine bleach you should use. You could try one cup of bleach mixed into a washing machine full of water. If the results are not light enough, you can repeat the process.
Please be careful when using either bleach or Fast Fade. Chlorine bleach is a very hazardous chemical; you should take care to avoid much exposure to it. Do not get it on your skin, and use good ventilation.
(Please help support this web site. Thank you.)
Message: I want to fade a new pair of extra large overalls. Can you tell me how much bleach to use in the washer? Should I add the bleach to the wait and let it agitate until mixed well before I add the overalls? I don't want them to be spotted.
Whatever discharge agent you use to lighten your overalls, you should certainly mix it well with the water in the washing machine before adding them. Or, you can mix it into a quart of water and add it to the automatic bleach dispenser on your washing machine, if there is one.
You have two main choices for chemicals to use to lighten the dye. The best choice would probably be Rit Fast Fade for Jeans, which is often available from fabric stores. Rit Fast Fade for Jeans contains sodium carbonate and sodium dichloroisocyanurate dihydrate. It is easier on the fabric than chlorine bleach, and is less apt to leave holes. Follow the instructions on the box.
The other choice is chlorine bleach, which contains sodium hypochlorite. Chlorine bleach will continue to eat away at the denim after you have finished using it, unless you neutralize it afterwards. You can neutralize it with a product called Anti-Chlor (sodium metabisulfite), or by rinsing with hydrogen peroxide or by washing with a chlorine-free oxygen 'bleach', such as OxyBoost. Do not attempt to use vinegar for this purpose, as the reaction between bleach and acid produces even more caustic and dangerous chemicals. I am not sure how much chlorine bleach you should use. You could try one cup of bleach mixed into a washing machine full of water. If the results are not light enough, you can repeat the process.
Please be careful when using either bleach or Fast Fade. Chlorine bleach is a very hazardous chemical; you should take care to avoid much exposure to it. Do not get it on your skin, and use good ventilation.
(Please help support this web site. Thank you.)
Tuesday, February 14, 2006
I noticed a spot on the chest of my sweater that appears lighter in color. If I would dye it black, would this spot cause a problem?
Name: Penny
Message: I have a dark beige sweater, 100% cotton. I noticed a spot on the chest that appears lighter in color. I don't know what happened. If I would dye it black, would this spot cause a problem? Thank you.
Yes, the lighter spot will probably still be lighter after you dye the sweater, though it may be less noticeable. See "How can I fix the bleach spots on my favorite clothing?".
By the way, it is best to avoid all-purpose dye when dyeing a cotton sweater. You will obtain better and longer-lasting results, without having to use hot water that might shrink the sweater, if you use fiber reactive dye, such as Procion MX dye. If you can't find this dye locally, you can order it by mail from any of the dye suppliers listed on my page of Sources for Dyeing Supplies.
(Please help support this web site. Thank you.)
Message: I have a dark beige sweater, 100% cotton. I noticed a spot on the chest that appears lighter in color. I don't know what happened. If I would dye it black, would this spot cause a problem? Thank you.
Yes, the lighter spot will probably still be lighter after you dye the sweater, though it may be less noticeable. See "How can I fix the bleach spots on my favorite clothing?".
By the way, it is best to avoid all-purpose dye when dyeing a cotton sweater. You will obtain better and longer-lasting results, without having to use hot water that might shrink the sweater, if you use fiber reactive dye, such as Procion MX dye. If you can't find this dye locally, you can order it by mail from any of the dye suppliers listed on my page of Sources for Dyeing Supplies.
(Please help support this web site. Thank you.)
Wednesday, February 08, 2006
What is the best fixative for directly applied deka batik dyes on silk
Name: Harold
Message: what is the best fixative for directly applied deka batik
dyes on silk...the instructions say fixative 111, but i cannot find anywhere to
purchase it...
Deka L dyes are not currently available in the US, but they are still available in other countries, and other brands of the same class of dyes are available. They are a type of all-purpose dye, which is a combination of hot-water acid dyes for wool and silk plus hot-water direct dyes for cotton. All-purpose dyes work best if simmered at 190°F. (87°C.) for half an hour, though this is impossible in batiking because the wax would melt. Although Deka L dyes can be used for batik, it is clear that any cold water dye would be much easier to use, more permanent, and all around superior for batik.
Deka L fixative is a proprietary formula, sold only where other products of the Deka company are sold. It is very likely, however, that it is similar to such products as Retayne and Raycafix; you can find Retayne at any good mail-order dye supplier or via a web search. Cationic dye fixatives such as Retayne must not be used until after the final stage of dyeing has been completed, because they may interfere with additional dyeing. They are useful in improving the washfastness of all-purpose dye so that it does not wash out as quickly, though they may decrease lightfastness.
I must recommend that you avoid the use of hot-water dyes for batik, even if you obtain a dye fixative such as Retayne. Cool water fiber reactive dyes are much more suitable, and can be used on silk with the same soda ash recipe that is used for cotton. The most popular type of fiber reactive dye is Procion MX dye, which readily available by mail order in the US, Canada, Australia, and Europe. You can order them from any of the dye suppliers around the world which I have listed on my Sources for Dyeing Supplies page.
(Please help support this web site. Thank you.)
Deka L dyes are not currently available in the US, but they are still available in other countries, and other brands of the same class of dyes are available. They are a type of all-purpose dye, which is a combination of hot-water acid dyes for wool and silk plus hot-water direct dyes for cotton. All-purpose dyes work best if simmered at 190°F. (87°C.) for half an hour, though this is impossible in batiking because the wax would melt. Although Deka L dyes can be used for batik, it is clear that any cold water dye would be much easier to use, more permanent, and all around superior for batik.
Deka L fixative is a proprietary formula, sold only where other products of the Deka company are sold. It is very likely, however, that it is similar to such products as Retayne and Raycafix; you can find Retayne at any good mail-order dye supplier or via a web search. Cationic dye fixatives such as Retayne must not be used until after the final stage of dyeing has been completed, because they may interfere with additional dyeing. They are useful in improving the washfastness of all-purpose dye so that it does not wash out as quickly, though they may decrease lightfastness.
I must recommend that you avoid the use of hot-water dyes for batik, even if you obtain a dye fixative such as Retayne. Cool water fiber reactive dyes are much more suitable, and can be used on silk with the same soda ash recipe that is used for cotton. The most popular type of fiber reactive dye is Procion MX dye, which readily available by mail order in the US, Canada, Australia, and Europe. You can order them from any of the dye suppliers around the world which I have listed on my Sources for Dyeing Supplies page.
(Please help support this web site. Thank you.)
Tuesday, February 07, 2006
Where can I buy blue Kool-Aid?

I  live in Clinton, MS and I can't buy blue koolaid here. I have been told
that you can't buy blue koolaid in the US. If you know of a source for it,
would you let me know.
live in Clinton, MS and I can't buy blue koolaid here. I have been told
that you can't buy blue koolaid in the US. If you know of a source for it,
would you let me know.
Apparently the Kool-Aid® company decided to market its blue drinks only in a form already pre-mixed with water. The blue lemonade, blue raspberry, and blue moon berry flavors no longer appear to be available in dry powdered form. Store brands have apparently followed suit, judging from the selection at my local grocer's. This might be regional.
Wool dyers can substitute Blue Food Coloring, available in the baking aisle of the grocery store. See "Using Food Coloring as a Textile Dye for Protein Fibers".

Kool-Aid enthusiasts can buy online, at least until supplies run out: see Kool-Aid Unsweetened Twist Blue Mountain Berry Drink Mix and Kool-Aid Unsweetened Island Twists Blue Raspberry Lemonade .
Another alternative is blue Wyler's Berry Jammer Drink Mix . This is in stock as of May 5, 2008.
. This is in stock as of May 5, 2008.
(Please help support this web site. Thank you.)
[Updated May 5, 2008.]
 live in Clinton, MS and I can't buy blue koolaid here. I have been told
that you can't buy blue koolaid in the US. If you know of a source for it,
would you let me know.
live in Clinton, MS and I can't buy blue koolaid here. I have been told
that you can't buy blue koolaid in the US. If you know of a source for it,
would you let me know.Apparently the Kool-Aid® company decided to market its blue drinks only in a form already pre-mixed with water. The blue lemonade, blue raspberry, and blue moon berry flavors no longer appear to be available in dry powdered form. Store brands have apparently followed suit, judging from the selection at my local grocer's. This might be regional.
Wool dyers can substitute Blue Food Coloring, available in the baking aisle of the grocery store. See "Using Food Coloring as a Textile Dye for Protein Fibers".
Kool-Aid enthusiasts can buy online, at least until supplies run out: see Kool-Aid Unsweetened Twist Blue Mountain Berry Drink Mix and Kool-Aid Unsweetened Island Twists Blue Raspberry Lemonade .
Another alternative is blue Wyler's Berry Jammer Drink Mix
(Please help support this web site. Thank you.)
[Updated May 5, 2008.]
Sunday, February 05, 2006
How to tie-dye yarmulkes
If someone was going to tye-dye yarmulkes, would it be better to
go with cotton, or with that shiny satiny type of
fabric?
Always choose cotton or silk, never polyester. Fiber content is everything; the specific weave (satin, twill, broadcloth, crocheted) doesn't matter a bit. All 100% natural fibers are good for dyeing. You use a different method for wool and other animal fibers. Hemp and linen are just like cotton, for dyeing.
50% cotton/50% polyester will *look* like 100% cotton, but when you dye it you get baby pastels instead of bright colors.
I'm sure it's far easier to find polyester satin than silk satin, in yarmulkes. Silk satin is so much nicer, but it's more expensive, and people don't even realize the difference unless it's pointed out to them. You can dye nylon, though. Just not any other synthetics. If the seller does not specify what the satin is made of, assume it is undyeable polyester.
You can draw on paper with special polyester dye crayons and then iron the designs onto polyester satin. Could be a fun project for kids. The polyester should be white for this purpose.
More questions....
Always choose cotton or silk, never polyester. Fiber content is everything; the specific weave (satin, twill, broadcloth, crocheted) doesn't matter a bit. All 100% natural fibers are good for dyeing. You use a different method for wool and other animal fibers. Hemp and linen are just like cotton, for dyeing.
50% cotton/50% polyester will *look* like 100% cotton, but when you dye it you get baby pastels instead of bright colors.
I'm sure it's far easier to find polyester satin than silk satin, in yarmulkes. Silk satin is so much nicer, but it's more expensive, and people don't even realize the difference unless it's pointed out to them. You can dye nylon, though. Just not any other synthetics. If the seller does not specify what the satin is made of, assume it is undyeable polyester.
You can draw on paper with special polyester dye crayons and then iron the designs onto polyester satin. Could be a fun project for kids. The polyester should be white for this purpose.
More questions....
0) Prewash: does it make any difference if the new kippot are washed
in hot or cold?
Hot is better, best to prewash in hot with soda ash and detergent. (The soda ash improves cleaning but does not substitute for the soda ash presoak. Soda ash rinses out easily.)
However, if the kippot need to be treated gently, just stick throughout to whatever care instructions you find best. I normally just throw my dyeables in the laundry. Try washing just one in hot, and if it shrinks out of shape, treat the rest with much more care!
Hot is better, best to prewash in hot with soda ash and detergent. (The soda ash improves cleaning but does not substitute for the soda ash presoak. Soda ash rinses out easily.)
However, if the kippot need to be treated gently, just stick throughout to whatever care instructions you find best. I normally just throw my dyeables in the laundry. Try washing just one in hot, and if it shrinks out of shape, treat the rest with much more care!
1) Color. My first thought was just to go with one color for the
entire kippah, since the things are small (~5 inches across). [My daughter]
wasn't enthralled with that--she likes the idea of more colors. Someone
suggested using different tones of the same color family (e.g. light, medium,
and dark blue) rather than the classic yellow/magenta/teal. I like this, since
it seems that the inevitable goofs won't be quite as jarring--easiest I'd guess
would be to use the same dye, and just add more or less dye than recommended to
weaken or strengthen the color. Would that work? Another thought I had
earlier was to dye the kippot one lighter color and then use the technique you'd
mentioned before of shaking pure dry dye particles on of a darker
color.
Avoid shaking dry dye in quantity - you don't want to breathe the stuff. I don't want kids using it.
I favor using several different colors that are close and look pretty together. Say, turquoise and cerulean and navy. I think the results are prettier and more complex than just using gradations of the same color. You will get gradations of each color due to the tying, anyway, with lighter areas near where the rubber bands go.
I would rather avoid using all three primaries in one little thing. If all three colors mix together, the result is not pretty! Pick two primaries and the secondary color that falls between them. This way you will be sure not to get mud. Say, turquoise and magenta and purple, or yellow and orange and magenta, or yellow and green and turquoise. Mix up two colors from dye powder than mix the intervening color by mixing those two.
It is often best to use pure single color dyes, not dye mixtures, since funny things can happen at the edges when mixed colors meet - see http://www.pburch.net/dyeing/FAQ/pureMXcolors.shtml
Avoid shaking dry dye in quantity - you don't want to breathe the stuff. I don't want kids using it.
I favor using several different colors that are close and look pretty together. Say, turquoise and cerulean and navy. I think the results are prettier and more complex than just using gradations of the same color. You will get gradations of each color due to the tying, anyway, with lighter areas near where the rubber bands go.
I would rather avoid using all three primaries in one little thing. If all three colors mix together, the result is not pretty! Pick two primaries and the secondary color that falls between them. This way you will be sure not to get mud. Say, turquoise and magenta and purple, or yellow and orange and magenta, or yellow and green and turquoise. Mix up two colors from dye powder than mix the intervening color by mixing those two.
It is often best to use pure single color dyes, not dye mixtures, since funny things can happen at the edges when mixed colors meet - see http://www.pburch.net/dyeing/FAQ/pureMXcolors.shtml
2) Curing/washing the little buggers. We're going to have an
assembly line of friends helping with the dyeing. Do we finish dyeing each one,
pop each into an individual baggie, and seal it up? And when it's time to untie
them a few hours later, does it make any difference if some of them have sat for
an additional hour or two? How about when I dump them into the synthrapol-laden
washing machine: can the first few sit in there for an hour before the final
ones make it into the machine?
What is the temperature going to be? If you use urea in the mix it will keep them moist; urea is a humectant and is traditional for tie-dyeing. You must let them react at 70 degrees F. or above though. If it is that warm, you do not need to wrap in plastic at all, as the urea will take care of maintaining adequate moisture. If it is too cold, you need to place them in a warm place, though, which generally means wrapping them up so you can move them. If their colors are compatible, instead of being all three primaries, you may be able to get away with popping several in a larger bag.
You can leave them overnight, or even two nights. It is unlikely to be a problem. Some people think that maybe leaving things to sit for several days may encourage the formation of small holes. Others say there is no relationship and the people with the holes just ordered some low quality blanks to dye, whose holes did not show up until after the washing out process.
I usually leave things until the next day to wash out., Doing so causes all of the dye to be done reacting, either with the fabric or the water, so no active dye remains to transfer to the wrong part of the item during washing. For tie-dyeing in which you want to minimize color changes after you have finished - no dark spots in the yellow sections - it is important to leave things to react for longer than necessary.
Before you dump things into the machine, be sure to fill it quite full with cold water and synthrapol. Try not to dump things in until you are getting ready to wash a load. I fill the machine and use children's blunt-ended scissors to snip rubber bands as I toss them in. I do not even prerinse at all, usually, since I am lazy. Don't use hot water until the second washing. Soaking in hot water makes for more efficient dye removal. We turn off the cold water to the washer because our washer insists on adding some cold water when we have it set to hot. After some minutes of agitation in hot water, I may turn off the machine for a while to allow a longer cycle, to take full advantage of the hot water. Usually at least two washings in hot water. More washings than that if you have to use warm water to avoid shrinkage.
Two other possibilities that are easier than multi-color tie-dyeing:
Single color tie-dye - tie them all up, then dye in the washing machine as indicated at
http://www.pburch.net/dyeing/FAQ/washingmachine.shtml
Do several batches, each a different color.
Low water immersion - wad up a bunch of them and stuff them in a bucket, then pour over two different colors of dye, enough to almost cover them, let rest for a bit, then pour on the soda ash. see
http://www.pburch.net/dyeing/lowwaterimmersion.shtml
Also see a couple of photos in my comment at
http://www.pburch.net/drupal/?q=node/30#comment-66
(Please help support this web site. Thank you.)
What is the temperature going to be? If you use urea in the mix it will keep them moist; urea is a humectant and is traditional for tie-dyeing. You must let them react at 70 degrees F. or above though. If it is that warm, you do not need to wrap in plastic at all, as the urea will take care of maintaining adequate moisture. If it is too cold, you need to place them in a warm place, though, which generally means wrapping them up so you can move them. If their colors are compatible, instead of being all three primaries, you may be able to get away with popping several in a larger bag.
You can leave them overnight, or even two nights. It is unlikely to be a problem. Some people think that maybe leaving things to sit for several days may encourage the formation of small holes. Others say there is no relationship and the people with the holes just ordered some low quality blanks to dye, whose holes did not show up until after the washing out process.
I usually leave things until the next day to wash out., Doing so causes all of the dye to be done reacting, either with the fabric or the water, so no active dye remains to transfer to the wrong part of the item during washing. For tie-dyeing in which you want to minimize color changes after you have finished - no dark spots in the yellow sections - it is important to leave things to react for longer than necessary.
Before you dump things into the machine, be sure to fill it quite full with cold water and synthrapol. Try not to dump things in until you are getting ready to wash a load. I fill the machine and use children's blunt-ended scissors to snip rubber bands as I toss them in. I do not even prerinse at all, usually, since I am lazy. Don't use hot water until the second washing. Soaking in hot water makes for more efficient dye removal. We turn off the cold water to the washer because our washer insists on adding some cold water when we have it set to hot. After some minutes of agitation in hot water, I may turn off the machine for a while to allow a longer cycle, to take full advantage of the hot water. Usually at least two washings in hot water. More washings than that if you have to use warm water to avoid shrinkage.
Two other possibilities that are easier than multi-color tie-dyeing:
Single color tie-dye - tie them all up, then dye in the washing machine as indicated at
http://www.pburch.net/dyeing/FAQ/washingmachine.shtml
Do several batches, each a different color.
Low water immersion - wad up a bunch of them and stuff them in a bucket, then pour over two different colors of dye, enough to almost cover them, let rest for a bit, then pour on the soda ash. see
http://www.pburch.net/dyeing/lowwaterimmersion.shtml
Also see a couple of photos in my comment at
http://www.pburch.net/drupal/?q=node/30#comment-66
(Please help support this web site. Thank you.)
Friday, February 03, 2006
I want to remove dye and redye solid embossed velvet. enough for a sofa an two chairs to be recovered.
Name: Donna
Message: I want to rmove dye and redye solid embossed velvet. enough for a sofa an two chairs to be recovered. how much color would I need to take what is now light salmon flesh color(very pale) from what was originally cayenne, with the pale pink what is the best color used to achieve cabarnet/ ruby color. or Rich purple/mauve , or a silver smoke / pewter (light to dark? What light pink would I use to match pewter and silver smoke has mauve grey hue What do you think of Dylon and Tintex and do you know of a wholesale rep in Canada?
What fiber are you using? Unfortunately, you simply cannot choose a dye without knowing the fiber content of your velvet. Velvet can be woven from many different fibers, both synthetic and natural, including silk, polyester, nylon, and many others.
Once you know your fiber content, you can select your dye. See the overview page "About the Dyes", which explains dye choice based on fiber content. If your velvet is made of cotton or rayon, I recommend fiber reactive dye, such as Procion MX dye. If your velvet is made out of silk or nylon, you can use acid dye. If it is made of polyester, I do not recommend that you even consider dyeing it, though you can use fabric paints.
You cannot remove dye from most synthetic fibers at all. It is impossible to predict whether dye can be discharged or removed from even natural fibers, because it all depends on the specific dye used by the manufacturer. Do not use chlorine bleach (hypochlorite) on any synthetic fiber whatsoever, nor on any animal-type fiber such as silk or wool, because it will destroy them; it
will also damage even the sturdiest cotton if used without great care. You may
use Color Remover (sodium hydrosulfate, sold as Rit brand Color Remover) on
cotton, silk, or nylon; you must be very careful, however, to avoid destroying
silk or nylon with Color Remover, and even so you might damage your velvet
badly. Do not use any treatment that violates the care instructions for your
fabric; if the label says 'dry clean only' do not attempt to wash it, remove
color from it, or dye it, and if it says to wash in cool water, do not use any
dye or treatment that requires hot water.
any animal-type fiber such as silk or wool, because it will destroy them; it
will also damage even the sturdiest cotton if used without great care. You may
use Color Remover (sodium hydrosulfate, sold as Rit brand Color Remover) on
cotton, silk, or nylon; you must be very careful, however, to avoid destroying
silk or nylon with Color Remover, and even so you might damage your velvet
badly. Do not use any treatment that violates the care instructions for your
fabric; if the label says 'dry clean only' do not attempt to wash it, remove
color from it, or dye it, and if it says to wash in cool water, do not use any
dye or treatment that requires hot water.
Dylon and Tintex are both companies that market many different types of dyes. Some of their dyes, such as Dylon Cold Water Dye, work well on cotton at room temperature. Some of their dyes, such as Dylon Multi Purpose, work only in nearly boiling water. Neither company sells any dye that will work on polyester. It is impossible to comment on these companies' dyes' suitability to a fabric of unknown fiber type.
(Please help support this web site. Thank you.)
Message: I want to rmove dye and redye solid embossed velvet. enough for a sofa an two chairs to be recovered. how much color would I need to take what is now light salmon flesh color(very pale) from what was originally cayenne, with the pale pink what is the best color used to achieve cabarnet/ ruby color. or Rich purple/mauve , or a silver smoke / pewter (light to dark? What light pink would I use to match pewter and silver smoke has mauve grey hue What do you think of Dylon and Tintex and do you know of a wholesale rep in Canada?
What fiber are you using? Unfortunately, you simply cannot choose a dye without knowing the fiber content of your velvet. Velvet can be woven from many different fibers, both synthetic and natural, including silk, polyester, nylon, and many others.
Once you know your fiber content, you can select your dye. See the overview page "About the Dyes", which explains dye choice based on fiber content. If your velvet is made of cotton or rayon, I recommend fiber reactive dye, such as Procion MX dye. If your velvet is made out of silk or nylon, you can use acid dye. If it is made of polyester, I do not recommend that you even consider dyeing it, though you can use fabric paints.
You cannot remove dye from most synthetic fibers at all. It is impossible to predict whether dye can be discharged or removed from even natural fibers, because it all depends on the specific dye used by the manufacturer. Do not use chlorine bleach (hypochlorite) on any synthetic fiber whatsoever, nor on
 any animal-type fiber such as silk or wool, because it will destroy them; it
will also damage even the sturdiest cotton if used without great care. You may
use Color Remover (sodium hydrosulfate, sold as Rit brand Color Remover) on
cotton, silk, or nylon; you must be very careful, however, to avoid destroying
silk or nylon with Color Remover, and even so you might damage your velvet
badly. Do not use any treatment that violates the care instructions for your
fabric; if the label says 'dry clean only' do not attempt to wash it, remove
color from it, or dye it, and if it says to wash in cool water, do not use any
dye or treatment that requires hot water.
any animal-type fiber such as silk or wool, because it will destroy them; it
will also damage even the sturdiest cotton if used without great care. You may
use Color Remover (sodium hydrosulfate, sold as Rit brand Color Remover) on
cotton, silk, or nylon; you must be very careful, however, to avoid destroying
silk or nylon with Color Remover, and even so you might damage your velvet
badly. Do not use any treatment that violates the care instructions for your
fabric; if the label says 'dry clean only' do not attempt to wash it, remove
color from it, or dye it, and if it says to wash in cool water, do not use any
dye or treatment that requires hot water.Dylon and Tintex are both companies that market many different types of dyes. Some of their dyes, such as Dylon Cold Water Dye, work well on cotton at room temperature. Some of their dyes, such as Dylon Multi Purpose, work only in nearly boiling water. Neither company sells any dye that will work on polyester. It is impossible to comment on these companies' dyes' suitability to a fabric of unknown fiber type.
(Please help support this web site. Thank you.)
Thursday, February 02, 2006
5th grade science project on what materials dye the best
Name: Jessica
Message: Hi I'm in the 5th grade and have to do a science project..I picked doing it on what materials dye the best can you help me with that...I was just typing in tye dye and saw your link it is cool..I don't really no how i came up with it and i really don't know how to do the project where to start..lol Can you help?
Comparing how dyes work on different materials is a good idea. It makes a big difference what kind of fabric you try to dye. For example, tie-dyeing a shirt that is 100% cotton will produce much brighter results than trying to dye a shirt that is 50% cotton and 50% polyester.
You are going to have to choose what kind of dye to use. A good dye for children to use is food coloring. You can buy unsweetened Kool-aid at the store and use it for dyeing. This dye will not work on cotton, and it will not work on synthetics such as acrylic yarn, but it will work well on wool. Go to the store and buy some wool yarn and some acrylic yarn, or else some wool fabric and some cotton fabric. You can buy as little as 1/4 yard of each type of fabric, or one ball of each type of yarn. You can use just a small piece of fabric, or a handful of yarn, in each test you do. Here is a good basic procedure to follow:
1. Get several microwave-safe bowls and in each one place the same amount of water.
2. Into this water mix one packet of the same color of Kool-aid (or another brand of artificially colored unsweetened drink mix).
3. Place a different type of yarn or small piece of fabric into each bowl, then have an adult help you with microwaving each bowl until the water just starts to boil.
4. Have the adult move each bowl to a heatproof surface (such as the top of the stove or several potholders).
5. Leave the bowls alone while they cool to room temperature, then rinse the dye out of each fabric or yarn sample. To thoroughly wash the fabric or yarn, you can use a net bag, such as is used for washing lingerie, and run them through the washing machine, or you can just wash thoroughly by hand at the sink. Use either dishwashing detergent or shampoo as the soap for washing out the excess dye.
6. You should be able to see from your results that some fibers dye much brighter than others with the same dye.
You can find some information to help explain your results on these pages on my web site:
How can I tie dye with Kool-aid?
What kinds of chemical bonds attach dyes to fibers?
Using Food Coloring as a Textile Dye for Protein Fibers
About Acid Dyes
Dyeing Protein Fibers
Note that food coloring is a type of acid dye, and wool is made of protein. If you have any questions after you do your experiments, please write me again.
You should also check your local public library to see whether you can find any good books on dyeing, for your bibliography. I especially recommend Linda Knutson's book, Synthetic Dyes for Natural Fibers, but your library might not have a copy of it. Unfortunately I have never seen a good book explaining dyeing for children. When my own children have done science fair projects with dyes, I have always had to help them find the information they wanted in books, and had to help them understand it.
If you decide later to learn to tie-dye cotton shirts, do not use food coloring or all-purpose dye. For dyeing cotton, you will be much happier with a tie-dye kit that contains Procion type dyes, such as the Jacquard brand tie-dye kit.
(Please help support this web site. Thank you.)
Message: Hi I'm in the 5th grade and have to do a science project..I picked doing it on what materials dye the best can you help me with that...I was just typing in tye dye and saw your link it is cool..I don't really no how i came up with it and i really don't know how to do the project where to start..lol Can you help?
Comparing how dyes work on different materials is a good idea. It makes a big difference what kind of fabric you try to dye. For example, tie-dyeing a shirt that is 100% cotton will produce much brighter results than trying to dye a shirt that is 50% cotton and 50% polyester.
You are going to have to choose what kind of dye to use. A good dye for children to use is food coloring. You can buy unsweetened Kool-aid at the store and use it for dyeing. This dye will not work on cotton, and it will not work on synthetics such as acrylic yarn, but it will work well on wool. Go to the store and buy some wool yarn and some acrylic yarn, or else some wool fabric and some cotton fabric. You can buy as little as 1/4 yard of each type of fabric, or one ball of each type of yarn. You can use just a small piece of fabric, or a handful of yarn, in each test you do. Here is a good basic procedure to follow:
1. Get several microwave-safe bowls and in each one place the same amount of water.
2. Into this water mix one packet of the same color of Kool-aid (or another brand of artificially colored unsweetened drink mix).
3. Place a different type of yarn or small piece of fabric into each bowl, then have an adult help you with microwaving each bowl until the water just starts to boil.
4. Have the adult move each bowl to a heatproof surface (such as the top of the stove or several potholders).
5. Leave the bowls alone while they cool to room temperature, then rinse the dye out of each fabric or yarn sample. To thoroughly wash the fabric or yarn, you can use a net bag, such as is used for washing lingerie, and run them through the washing machine, or you can just wash thoroughly by hand at the sink. Use either dishwashing detergent or shampoo as the soap for washing out the excess dye.
6. You should be able to see from your results that some fibers dye much brighter than others with the same dye.
You can find some information to help explain your results on these pages on my web site:
How can I tie dye with Kool-aid?
What kinds of chemical bonds attach dyes to fibers?
Using Food Coloring as a Textile Dye for Protein Fibers
About Acid Dyes
Dyeing Protein Fibers
Note that food coloring is a type of acid dye, and wool is made of protein. If you have any questions after you do your experiments, please write me again.
You should also check your local public library to see whether you can find any good books on dyeing, for your bibliography. I especially recommend Linda Knutson's book, Synthetic Dyes for Natural Fibers, but your library might not have a copy of it. Unfortunately I have never seen a good book explaining dyeing for children. When my own children have done science fair projects with dyes, I have always had to help them find the information they wanted in books, and had to help them understand it.
If you decide later to learn to tie-dye cotton shirts, do not use food coloring or all-purpose dye. For dyeing cotton, you will be much happier with a tie-dye kit that contains Procion type dyes, such as the Jacquard brand tie-dye kit.
(Please help support this web site. Thank you.)
